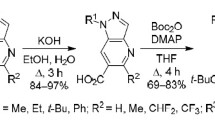
N-Boc-4-aminopyrazole-5-carbaldehydes reacted with aryl, hetaryl, alkyl, and cycloalkyl ketones containing methylene groups. The reactions were accomplished in refluxing acetic acid in the presence of pyrrolidine and resulted in the formation of 5-substituted and carbo[b]fused pyrazolo[4,3-b]pyridines.
Similar content being viewed by others
References
(а) Friedländer, P. Chem. Ber. 1882, 15, 2572. a Hassner, A.; Stumer, C. Organic Synthesis Based on Name Reaction; Pergamon: London, 2002, p. 118. b Marco-Contelles, J.; Pérez-Mayoral, E.; Samadi, A.; do Carmo Carreiras, M.; Soriano, E. Chem. Rev. 2009, 109, 2652.
(a) Cheng, C.-C.; Yan, S.-J. Org. React. 1982, 28, 37. (b) Coffey, D. S.; May, S. A.; Ratz, A. M. In Progress in Heterocyclic Chemistry; Gribble, W. G.; Gilchrist, T. L., Eds.; Pergamon: London 2001, Vol. 13, p. 238. (c) Pflum, D. A. In Name Reactions in Heterocyclic Chemistry; Li, J. J., Ed.; Wiley: Hoboken, 2005, p. 411.
Yang, D.; Jiang, U.; Li, J.; Xu, F. Tetrahedron 2007, 63, 7654.
(a) Litvinov, V. P. Russ. Chem. Rev. 2004, 73, 637. [Usp. Khim. 2004, 73, 692.] (b) Litvinov, V. P. Adv. Heterocycl. Chem. 2006, 91, 189. a Madaan, A.; Verma, R.; Kumar, V.; Singh, A. T.; Jain, S. K.; Jaggi, M. Arch. Pharm. 2015, 348, 837. b Fadda, A. A.; El-Hadidy, S. A.; Elattar, K. M. Synth. Commun. 2015, 45, 2765.
Perandones, F.; Soto, D. L. J. Heterocycl. Chem. 1997, 34, 107.
Yamanaka, H.; Sakamoto, T.; Shiozawa, A. Heterocycles 1977, 7, 51.
L’abbe, G.; Vandendriessche, A.; Weyns, N. Bull. Soc. Chim. Belg. 1988, 97, 85.
(a) Ahluwalia, V. K.; Gogal, B. Synth. Commun. 1996, 26, 1341. (b) Piao, M.-Z.; Ymafuku, K. J. Heterocycl. Chem. 1996, 33, 389. (c) Jachak, M. N.; Avhale, A. B.; Tantak, C. D.; Toche, R. B.; Reidlinger, C.; Stadlbauer, W. J. Heterocycl. Chem. 2005, 42, 1311. (d) Zheng, A.; Zhang, W.; Pan, J. Synth. Commun. 2006, 36, 1549. (e) Jackak, M. N.; Avhale, A. B.; Toche, R. B.; Sabnis, R. W. J. Heterocycl. Chem. 2007, 44, 343. (f) El-Emary, T. I. J. Chin. Chem. Soc. 2007, 54, 507. (g) Toche, R. B.; Bhavsar, D. C.; Kazi, M. A.; Bagul, S. M.; Jackak, M. N. J. Heterocycl. Chem. 2010, 47, 287.
Panda, N.; Karmakar, S.; Jena, A. K. Chem. Heterocycl. Compd. 2011, 46, 1500. [Khim. Geterotsikl. Compd. 2010, 1857.]
Barreiro, E. J.; Camara, C. A.; Verli, H.; Brazil-Más, L.; Castro, N. G.; Cintra, W. M.; Aracava, Y.; Rodrigues, C. R.; Fraga, C. A. J. Med. Chem. 2003, 46, 1144.
Laborde, E.; Macsata, R. W.; Meng, F.; Peterson, B. T.; Robinson, L.; Schow, S. R.; Simon, R. J.; Xu, H.; Baba, K.; Inagaki, H.; Ishiwata, Y.; Jomori, T.; Matsumoto, Y.; Miyachi, A.; Nakamura, T.; Okamoto, M.; Handel, T. M.; Bernard, C. C. A. J. Med. Chem. 2011, 54, 1667.
(a) Li, A.-H.; Ahmed, E.; Chen, X.; Cox, M.; Crew, A. P.; Dong, H.-Q.; Jin, M.; Ma, L.; Panicker, B.; Siu, K. W.; Steinig, A. G.; Stolz, K. M.; Tavares, P. A. R.; Volk, B.; Weng, Q.; Werner, D.; Mulvihill, M. J. Org. Biomol. Chem. 2007, 5, 61. (b) Li, A.-H.; Beard, D. J.; Coate, H.; Honda, A.; Kadalbajoo, A.; Kleinberg, A.; Laufer, R.; Mulvihill, K. M.; Nigro, A.; Rastogi, P.; Sherman, D.; Siu, K. W.; Steinig, A. G.; Wang, T.; Werner, D.; Crew, A. P.; Mulvihill, M. J. Synthesis 2010, 1678.
a Tucker, T. J.; Sisko, J. T.;Tynebor, R. M.; Williams, T. M.; Felock, P. J.; Flynn, J. A.; Lai, M. T.; Liang, Y.; McGaughey, G.; Liu, M.; Miller, M.; Moyer, G.; Munshi, V.; Perlow-Poehnelt, R.; Prasad, S.; Reid, J. C.; Sanchez, R.; Torrent, M.; Vacca, J. P.; Wan, B.-L.; Yan, Y., J. Med. Chem. 2008, 51, 6503. (b) Tite, T.; Lougiakis, N.; Skaltsounis, A.-L.; Marakos, P.; Pouli, N.; Tenta, R.; Balzarini, J. Synlett 2009, 1741.
(a) Herdemann, M.; Heit, I.; Bosch, F.-U.; Quintini, G.; Scheipers, C.; Weber, A. Bioorg. Med. Chem. Lett. 2010, 20, 6998. (b) Pryde, D. C.; Marron, B. E.; West, C. W.; Reister, S.; Amato, G.; Yoger, K.; Antonio, B.; Padilla, K.; Cox, P. J.; Turner, J.; Warmus, J. S.; Swain, N. A.; Omoto, K.; Mahoney, J. H.; Gerlach, A. C. ACS Med. Chem. Lett. 2017, 8, 666.
(a) Czodrowski, P.; Mallinger, A.; Wienke, D.; Esdar, C.; Pöschke, O.; Busch, M.; Rohdich, F.; Eccles, S. A.; OrtizRuiz, M.-J.; Schneider, R.; Florence, I. R.; Clarke, P. A.; Musil, D.; Schwarz, D.; Dale, T.; Urbahns, K.; Blagg, J.; Schiemann, K. J. Med. Chem. 2016, 59, 9337. (b) Lin, H.; Yamashita, D. S.; Zeng, J.; Xie, R.; Verma, S.; Luengo, J. I.; Rhodes, N.; Zhang, S.; Robell, K. A.; Choudhry, A. E.; Lai, Z.; Kumar, R.; Minthorn, E. A.; Brown, K. K.; Heerding, D. A. Bioorg. Med. Chem. Lett. 2010, 20, 679.
Yoshizawa, H.; Kubota, T.; Itani, H.; Ishitobi, H.; Miwa, H.; Nishitani, Y. Bioorg. Med. Chem. 2004, 12, 4211.
(a) Chelucci, G.; Orrù, G. Tetrahedron Lett. 2005, 46, 3493. (b) Chelucci, G. Chem. Soc. Rev. 2006, 35, 1230.
(a) Siu, T.; Liang, J.; Arruda, J.; Li, Y.; Jones, R. E.; DefeoJones, D.; Barnett, S. F.; Robinson, R. G. Bioorg. Med. Chem. Lett. 2008, 18, 4186. (b) Vu, C. B.; Casaubon, R. WO Patent 2010101949. b Chan, L.; Jin, H.; Stefanac, T.; Lavallée, J.-F.; Falardeau, G.; Bedard, J.; May, S.; Yuen, L. J. Med. Chem. 1999, 42, 3023. c Chan, L.; Jin, H.; Stefanac, T.; Wang, W.; Lavallée, J.-F.; Bédard, J.; May, S. Bioorg. Med. Chem. Lett. 1999, 9, 2583.
Yamanaka, T.; Ohki, H.; Ohgaki, M.; Okuda, S.; Toda, A.; Kawabata, K.; Inoue, S.; Misumi, K.; Itoh, K.; Satoh, K. WO Patent 2004101571.
(а) Muchowski, J. M.; Maddox, M. L. Can. J. Chem. 2004, 82, 461. a Makarov, A. S.; Sorotskaya L. N.; Uchuskin, M. G.; Trushkov, I. V. Chem. Heterocycl. Compd. 2016, 52, 1087. [Khim. Geterotsikl. Compd. 2016, 52, 1087.]
Rowbottom, M. W.; Hutchinson, J. H. WO Patent 201715221.
Bleicher, K.; Flohr, A.; Groebke-Zbinden, K.; Koerner, M.; Kuhn, B.; Peters, J.-U.; Rodriguez-Sarmiento, R. M.; Vieira, E. US Patent 2011183979.
Lin, Y.; Yang, X.; Pan, W.; Rao, Y. Org. Lett. 2016, 18, 2304.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(4/5), 379–385
Rights and permissions
About this article
Cite this article
Yakovenko, G.G., Lukianov, O.A., Bol’but, A.V. et al. N-Boc-4-aminopyrazole-5-carbaldehydes in Friendländer synthesis of pyrazolo[4,3-b]pyridines. Chem Heterocycl Comp 55, 379–385 (2019). https://doi.org/10.1007/s10593-019-02468-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02468-8