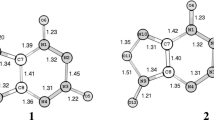
Thermolysis of 6-aryl-1,5-diazabicyclo[3.1.0]hexanes in methanol leads to the respective dimers, 5,11-diaryltetrahydro-1H,7Hdipyrazolo[1,2-a:1',2'-d]tetrazines. X-ray structural analysis of these compounds reveals the perhydrotetrazine ring adopting a chair conformation with the equatorially fused five-membered rings and with the equatorial orientation of the aryl substituents. A hindered rotation of the aryl substituents around the C–Ar bonds was observed by low-temperature NMR. Another NMR-observed process that takes place at low temperature is a reversible isomerization of the major eeee conformer into a slightly less stable aeee isomer. Density functional theory calculations (B3LYP/6-31+G(d)) on the conformational transformations of the parent tricyclic perhydrotetrazine and its 5,11-diphenyl derivative are in good agreement with the experimental findings and reveal that a conformation with the orthogonal orientation of the planes of the aryl substituents in respect to the average plane of the six-membered ring has the lowest energy.




Similar content being viewed by others
References
Makhova, N. N.; Shevtsov, A. V.; Petukhova, V. Yu. Russ. Chem. Rev. 2011, 80, 1035. [Usp. Khim. 2011, 80, 1087.]
Koptelov, Yu. B.; Kim, M. Kh.; Molchanov, A. P.; Kostikov, R. R. Russ. J. Org. Chem. 1999, 35, 110. [Zh. Org. Khim. 1999, 35, 116.]
Kornilova, T. A.; Kostikov, R. R.; Khlebnikov, A. F.; Zenkevich, I. G. J. Phys. Org. Chem. 2018, 31(7), e3843. DOI: https://doi.org/10.1002/poc.3843.
Koptelov, Yu. B. Russ. J. Org. Chem. 2006, 42, 1510. [Zh. Org. Khim. 2006, 42, 1524.]
Syroeshkina, Yu. S.; Kuznetsov, V. V.; Lyssenko, K. A.; Makhova, N. N. Russ. Chem. Bull., Int. Ed. 2009. 58, 366. [Izv. Akad. Nauk, Ser. Khim. 2009, 362.]
(a) Nelsen. S. F.; Hintz, P. J. J. Am. Chem. Soc. 1972, 94, 3138. (b) Baker, V. J.; Katritzky, A. R.; Majoral, J.-P.; Martin, A. R.; Sullivan, J. M. J. Am. Chem. Soc. 1976, 98, 5748. (c) Katritzky, A. R.; Ferguson, I. J.; Patel, R. C. J. Chem. Soc., Perkin Trans. 2 1979, 981.
Koptelov, Yu. B.; Kostikov, R. R.; Molchanov, A. P.; Kopf, J. Russ. J. Org. Chem. 1999, 35, 144. [Zh. Org. Khim. 1999, 35, 149].
Katritzky, A. R.; Baker, V. J.; Camalli, M.; Spagna, R.; Vaciago, A. J. Chem. Soc., Perkin Trans. 2 1980, 1733.
Ansell, G. B.; Erickson; J. L. J. Chem. Soc., Perkin Trans. 2 1975, 270.
Spagna, R.; Vaciago, A. Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem. 1978, 34B, 993.
(a) Akhmetova, V. R.; Nadyrgulova, G. R.; Tyumkina, T. V.; Starikova, Z. A.; Golovanov, D. G.; Antipin, M. Yu.; Kunakova, R. V.; Dzhemilev, U. M. Russ. Chem. Bull., Int. Ed. 2006, 55, 1824. [Izv. Akad. Nauk, Ser. Khim. 2006, 1758.] (b) Matsuyama, T.; Seguchi, K. Anal. Sci.: X-Ray Struct. Anal. Online 2007, 23, x87.
(a) Rademacher, P.; Koopman, H. Chem. Ber. 1975, 108, 1557. (b) Rademacher, P.; Breier, H.; Poppek, R. Chem. Ber. 1979, 112, 853. (c) Katritzky, A. R.; Baker, V. J.; Brito-Palma, F. M. S.; Patel, R. C.; Pfister-Guillouzo, G.; Guimon, C. J. Chem. Soc., Perkin Trans. 2 1980, 91.
(a) Palatinus, L.; Chapuis, G. J. Appl. Crystallogr. 2007, 40, 786. (b) Palatinus, L.; van der Lee, A. J. Appl. Crystallogr. 2008, 41, 975. (c) Palatinus, L. Prathapa, S. J.; van Smaalen, S. J. Appl. Crystallogr. 2012, 45, 575.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16, Revision A.03; Gaussian, Inc.; Wallingford, 2016.
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (grant № 15-03-05319).
NMR, HRMS, and XRD analyses were performed at the Saint Petersburg State University Center for Magnetic Resonance, Center for Chemical Analysis and Materials Research, and X-ray Diffraction Center, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
The Supplementary information file containing 1H and 13C{1H} NMR spectra is available at the journal website at http://link.springer.com/journal/10593.
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(2), 172–177
Rafael R. Kostikov is deceased
Electronic supplementary material
ESM 1
(PDF 1146 kb)
Rights and permissions
About this article
Cite this article
Kostikov, R.R., Kornilova, T.A., Khlebnikov, A.F. et al. Spatial Structure and Nontrivial Stereodynamics of Tricyclic Perhydro-1,2,4,5-Tetrazines. Chem Heterocycl Comp 55, 172–177 (2019). https://doi.org/10.1007/s10593-019-02435-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02435-3