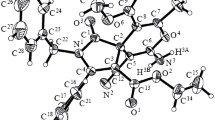
5-Aryl-2-carboxy-5-(pyridin-1-ium-1-yl)penta-2,4-dienoates, previously unknown pyridine betaines, were formed in 32–70% yields as a result of the reaction of arylpropynals, substituted pyridines, and malonic acid instead of the expected 5-arylpent-2-en-4-ynoic acids. The molecular structure of the novel compounds was studied by X-ray structural analysis.

Similar content being viewed by others
References
Grigoraş, A. G.; Dunca, S. I.; Ochiuz, L. Farmacia (Bucharest, Rom.) 2017, 65, 104.
Gibbons, H.; Michielsen, C. J. R.; Rundle, M.; Frost, G.; McNulty, B. A.; Walton, J.; Flynn, A.; Gibney, M. J.; Brennan, L. Mol. Nutr. Food Res. 2017, 61, DOI: https://doi.org/10.1002/mnfr.201700037.
Sakai, R.; Suzuki, K.; Shimamoto, K.; Kamiya, H. J. Org. Chem. 2004, 69, 1180.
Day, C. R.; Kempson, S. A. Biochim. Biophys. Acta, Gen. Subj. 2016, 1860, 1098.
(a) Wang, L.; Asthagiri, D.; Zeng, Y.; Chapman, W. G. Energy Fuels 2017, 31, 1512. (b) Song, B.; Hu, X.; Shui, X.; Cui, Z.; Wang, Z. Colloids Surf., A 2016, 489, 433. (c) Teo, S. H.; Islam, A.; Masoumi, H. R. F.; Taufiq-Yap, Y. H.; Janaun, J.; Chan, E.-S.; Khaleque, M. A. Renewable Energy 2017, 111, 892.
Kreicberga, J.; Laipniece, L.; Bērziņa, G.; Kampars, V. Chem. Heterocycl. Compd. 2010, 46, 438. [Khim. Geterotsikl. Soedin. 2010, 551.]
Xu, J.; Zhang, B.; Jansen, M.; Goerigk, L.; Wong, W. H. W.; Ritchie, C. Angew. Chem., Int. Ed. 2017, 56, 13882.
(a) Reissig, H.-U.; Domínguez, M. Chem. Select. 2016, 1, 5270. (b) Machado, V. G.; Stock, R. I.; Reichardt, C. Chem. Rev. 2014, 114, 10429.
Wiley, R. H.; Jarboe, C. H.; Hayes, F. N. J. Am. Chem. Soc. 1957, 79, 2602.
(a) Golovanov, A. A.; Odin, I. S.; Vologzhanina, A. V.; Bekin, V. V.; Nebritova, A. E. Russ. J. Org. Chem. 2014, 50, 943. [Zh. Org. Khim. 2014, 963.] (b) Golovanov, A. A.; Bekin, V. V.; Odin, I. S.; Chertov, A. Yu.; Grigor'eva, O. B.; Pisareva, V. S. Russ. J. Org. Chem. 2015, 51, 1688. [Zh. Org. Khim. 2015, 1723.]
Zhu, C.-Z.; Sun, Y.-L.; Wei, Y.; Shi, M. Adv. Synth. Catal. 2017, 359, 1263.
Ni, S.; Chen, J.; Ma, S. Org. Lett. 2013, 15, 3290.
Kim, T.; Al-Muhanna, M. K.; Al-Suwaidan, S. D.; Al-Kaysi, R. O.; Bardeen, C. J. Angew. Chem., Int. Ed. 2013, 52, 6889.
(a) Gusev, D. M.; Bormotin, А. А.; Rakshin, S. O.; Mel’nikov, P. А.; Raskil’dina, G. Z.; Chanishev, R. R.; Golovanov, А. А. Bashkir. khim. zhurn. 2018, 25, 90. (b) Golovanov, A. A.; Raskil'dina, G. Z.; Bekin. V. V.; Bunev, A. S.; Mikhailova, N. N.; Raskildina, G. Z.; Zlotskii S. S. Russ. Chem. Bull., Int. Ed. 2016. 65, 1757. [Izv. Akad. Nauk, Ser. Khim. 2016, 1757.]
Reddy, S. R.; Chadha A. RSC Adv. 2013, 3, 14929.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, A71, 3.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschman, H. J. Appl. Crystallogr. 2009, 42, 339.
The study was supported by the Russian Science Foundation (grant 18-13-00008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(1), 93–96
Rights and permissions
About this article
Cite this article
Golovanov, A.А., Dan’kov, S.А., Sokov, S.А. et al. An unusual result of the reaction of α-acetylene aldehydes, pyridines, and malonic acid. Synthesis and structure of novel pyridine betaines. Chem Heterocycl Comp 55, 93–96 (2019). https://doi.org/10.1007/s10593-019-02424-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02424-6