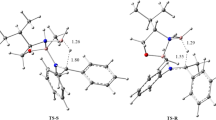
The molecular mechanism of nitrous acid elimination from 5-nitro-3-phenyl-4,5-dihydroisoxazole systems has been explored using DFT computational study. It was found that under both catalytic and non-catalytic conditions, these processes proceed according to a one-step asynchronous mechanism. The asynchronicity of transition state is determined by presence of Lewis acid catalyst. However, it is not enough to force an ionic mechanism. The proposed pattern can be considered as a general mechanism for this group of heterocyclic compounds.

Similar content being viewed by others
References
Varvounis, G.; Gerontitis, I. E.; Gkalpinos, V. Chem. Heterocycl. Compd. 2018, 54, 249. [Khim. Geterotsikl. Soedin. 2018, 54, 249.]
Markitanov, Yu. N.; Timoshenko, V. M.; Shermolovich, Yu. G. Chem. Heterocycl. Compd. 2018, 54, 89. [Khim. Geterotsikl. Soedin. 2018, 54, 89.]
Sysak, A.; Obmińska-Mrukowicz, B. I. Eur. J. Med. Chem. 2017, 137, 292.
Zhang, H.-Z.; Zhao, Z.-L.; Zhou, C.-H. Eur. J. Med. Chem. 2018, 144, 444.
Carruthers, W. Cycloaddition Reactions in Organic Synthesis; Pergamon: Richmond, 1990.
Advances in Quantum Chemistry; Sabin, J., Brandas, E., Eds.; Academic Press, 1985.
Muhlstadt, V. M.; Schulze, B. J. Prakt. Chem. 1971, 313, 745.
Koroleva, E. V.; Bondar, N. F.; Katok, Ya. M.; Chekanov, N. A.; Chernikhova, T. V. Chem. Heterocycl. Compd. 2007, 43, 362. [Khim. Geterotsikl. Soedin. 2007, 447.]
Jasiński, R.; Jasińska, E.; Dresler, E. J. Mol. Model. 2017, 23, 13.
Zhao, Y.; Truhlar, D. G. Acc. Chem. Res. 2008, 41, 157.
Kącka, A.; Jasiński, R. Comput. Theor. Chem. 2017, 1104, 37.
Kącka, A.; Domingo, L. R.; Jasiński, R. Res. Chem. Intermed. 2018, 44, 325.
Domingo, L. R. RSC Adv. 2014, 4, 32415.
Lewis Acids in Organic Synthesis; Yamamoto, H., Ed.; WILEY-VCH Verlag GmbH, 2008.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 16, Revision A.03; Gaussian Inc.: Wallingford, 2016.
Ndassa, I. M.; Adjieufack, A. I.; Ketcha, J. M.; Berski, S.; Ríos-Gutierrez, M.; Domingo, L. R. Int. J. Quantum Chem. 2017, 117, e25451.
Jasiński, R. Comput. Theor. Chem. 2014, 1046, 93.
Jasiński, R. J. Fluorine Chem. 2014, 160, 29.
Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. J. Comput. Chem. 2003, 24, 669.
All calculations reported in this manuscript were performed on Prometheus cluster in the CYFRONET regional computational center in Cracow.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(12), 1172–1174
Rights and permissions
About this article
Cite this article
Łapczuk-Krygier, A., Jaśkowska, J. & Jasiński, R. The influence of Lewis acid catalyst on the kinetic and molecular mechanism of nitrous acid elimination from 5-nitro-3-phenyl-4,5-dihydroisoxazole: DFT computational study. Chem Heterocycl Comp 54, 1172–1174 (2018). https://doi.org/10.1007/s10593-019-02410-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02410-y