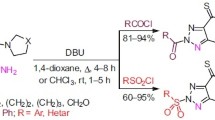
A three-step method was designed and developed on the basis of retrosynthetic analysis for the synthesis of hybrid molecules containing a thiazole ring and an N-sulfonyl amidine fragment, connected by a methylene linker. The mechanism of the last step involves the formation of intermediate 1,2,3,4-thiatriazoles and their transformation into the final products as a result of the elimination of molecular nitrogen and sulfur.

Similar content being viewed by others
References
(a) Ayati, A.; Emami, S.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Eur. J. Med. Chem. 2015, 97, 699. (b) Campiglia, P.; Scrima, M.; Grimaldi, M.; Cioffi, G.; Bertamino, A.; Sala, M.; Aquino, C.; Gomez-Monterrey, I.; Grieco, P.; Novellino, E.; D'Ursi, A. M. Chem. Biol. Drug Des. 2009, 74, 224. (c) Helal, M. H. M.; Salem, M. A.; El-Gaby, M. S. A.; Aljahdali, M. Eur. J. Med. Chem. 2013, 65, 517. (d) Romagnoli, R.; Baraldi, P. G.; Salvador, M. K.; Camacho, M. E.; Preti, D.; Tabrizi, M. A.; Bassetto, M.; Brancale, A.; Hamel, E.; Bortolozzi, R.; Basso, G.; Viola, G. Bioorg. Med.Chem. 2012, 20, 7083. (e) Naveena, C. S.; Poojary, B.; Arulmoli, T.; Manjunatha, K.; Prabhu, A.; Kumari, N. S. Med. Chem. Res. 2013, 22, 1925. (f) Yavari, I.; Malekafzali, A.; Seyfi, S. J. Iran. Chem. Soc. 2014, 11, 285. (g) Ibrar, A.; Tehseen, Y.; Khan, I.; Hameed, A.; Saeed, A.; Furtmann, N.; Bajorath, J.; Iqbal, J. Bioorg. Chem. 2016, 68, 177.
(a) Mahmoodi, N. O.; Parvizi, J.; Sharifzadeh, B.; Rassa, M. Arch. Pharm. 2013, 346, 860. (b) Denisova, A. B.; Sosnovskikh, V. Ya.; Dehaen, W.; Toppet, S.; Van Meervelt, L.; Bakulev, V. A. J. Fluorine Chem. 2002, 115, 183. (c) Korol, N. I.; Slivka, M. V. Chem. Heterocycl. Compd. 2017, 53, 852. [Khim. Geterotsikl. Soedin. 2017, 53, 852.]
(a) Lee, M. Y.; Kim, M. H.; Kim, J.; Kim, S. H.; Kim, B. T.; Jeong, I. H.; Chang, S.; Kim, S. H.; Chang, S.-Y. Bioorg. Med. Chem. Lett. 2010, 20, 541. (b) Beryozkina, T.; Bakulev, V.; Dianova, L.; Berseneva, V.; Slepukhin, P.; Leban, J.; Kalaba, P.; Aher, N. Y.; Ilic, M.; Sitte, H. H.; Lubec, G. Synthesis 2016, 1046. (c) Rupakova, N. A.; Bakulev, V. A.; Knippschild, U.; García-Reyes, B.; Eltsov, O. S.; Slesarev, G. P.; Beliaev, N. A.; Slepukhin, P. A.; Witt, L.; Peifer, C.; Beryozkina, T. V. ARKIVOC 2017, (iii), 225.
Chhabria, M. T.; Patel, S.; Modi, P.; Brahmkshatriya, P. S. Curr. Top. Med. Chem. 2016, 16, 2841.
Cummings, C. G.; Hamilton, A. D. Tetrahedron 2013, 69, 1663.
Jagodzinski, T. S. Chem. Rev. 2003, 103, 197.
(a) Dyachenko, V. D.; Dyachenko, I. V.; Nenajdenko, V. G. Rus. Chem. Rev. 2018, 87, 1. [Usp. Khim. 2018, 87, 1.] (b) Bakulev, V. A.; Lebedev, A. T.; Dankova, E. F.; Mokrushin, V. S.; Petrosyan, V. S. Tetrahedrоn 1989, 45, 7329. a Morzherin, Yu. Yu.; Kosterina, M. F.; Berseneva, V. S.; Dehaen, W.; Bakulev, V. A. Russ. Chem. Bull., Int. Ed. 2002, 51, 1292. [Izv. Akad. Nauk, Ser. Khim. 2002, 1194.]
(a) Belskaia, N. P.; Deryabina, T. G.; Koksharov, A. V.; Kodess, M. I.; Dehaen, W.; Lebedev, A. T; Bakulev, V. A. Tetrahedron Lett. 2007, 48, 9128. (b) Baeten, M.; Maes, B. U. W. Adv. Synth. Catal. 2016, 358, 826.
Hong, D. J.; Baek, J. O.; Oh, H. S.; Lee, M. S.; Ha, T. H.; Suh, K. H. WO Patent 2015152550.
Yavari, I.; Ahmadian, S.; Ghazanfarpur-Darjani, M.; Solgi, Y. Tetrahedron Lett. 2011, 52, 668.
(а) Efimov, I.; Beliaev, N.; Beryozkina, T.; Slepukhin, P.; Bakulev, V. Tetrahedron Lett. 2016, 57, 1949. a Efimov, I.; Bakulev, V.; Beliaev, N.; Beryozkina, T.; Knippschild, U.; Leban, J.; Zhi-Jin, F.; Eltsov, O.; Slepukhin, P.; Ezhikova, M.; Dehaen, W. Eur. J. Org. Chem. 2014, 3684.
Bakulev, V. A.; Beryozkina, T.; Thomas, J.; Dehaen, W. Eur. J. Org. Chem. 2018, 3, 262.
Filimonov, V. O.; Dianova, L. N.; Galata, K. A.; Beryozkina, T. V.; Novikov, M. S.; Berseneva, V. S.; Eltsov, O. S.; Lebedev, A. T.; Slepukhin, P. A.; Bakulev, V. A. J. Org. Chem. 2017, 82, 4056.
Aswad, M.; Chiba, J.; Tomohiro, T.; Hatanaka, Y. Chem. Commun. 2013, 49, 10242.
Dianova, L.; Berseneva, V.; Beryozkina, T.; Efimov, I.; Kosterina, M.; Eltsov, O.; Dehaen, W.; Bakulev, V. Eur. J. Org. Chem. 2015, 6917.
(a) Bakulev, V. A.; Morzherin, Y. Y.; Lebedev, A. T.; Dankova, E. F.; Kolobov, M. Y.; Shafran, Y. M. Bull. Soc. Chem. Belg. 1993, 102, 493. (b) Fabian, W. M. F.; Bakulev, V. A.; Kappe, C. O. J. Org. Chem. 1998, 63, 5801. (с) Bakulev, V. A. Russ. Chem. Rev. 1995, 64, 99. [Usp. Khim. 1995, 64, 99.]
Schaper, W. Synthesis 1985, 861.
Cambeiro, X. C.; Pericas, M. A. Adv. Synth. Catal. 2011, 353, 113.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
This work was supported by the Russian Foundation for Basic Research (grant No. 17-03-00641 / 17).
X-ray diffraction study was carried out on the equipment of the Center for Spectroscopy and Analysis of Organic compounds of the Postovsky Institute of Organic Synthesis of Ural Branch of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(12), 1153–1160
Rights and permissions
About this article
Cite this article
Il’kin, V.G., Berseneva, V.S., Slepukhin, P.А. et al. An efficient method for the synthesis of 2-thiazoleacetic acid N-sulfonyl amidines. Chem Heterocycl Comp 54, 1153–1160 (2018). https://doi.org/10.1007/s10593-019-02407-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02407-7