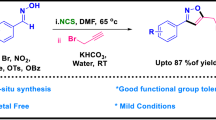
A simple one-pot two-stage method for the synthesis of disubstituted 4-chloro-, 4-bromo-, and 4-iodo-1,2,3-triazoles from terminal alkynes and organic azides is proposed.
Similar content being viewed by others
References
Duan, T.; Fan, K.; Fu, Y.; Zhong, C.; Chen, X.; Peng, T.; Qin, J . Dyes Pigm. 2012, 94, 28.
Wang, Z.; Tao, Y.; Wang, Z.; Yan, J. Polym. Chem. 2016, 7, 3172.
Lee, D.-H.; Kim, K. T.; Jang, Y.; Lee, S.; Jeon, H. B.; Paik, H.-J.; Min, B. S.; Kim, W. J. Appl. Polym. Sci. 2014, 131, DOI:https://doi.org/10.1002/app.40594.
Chen, Z.; Zheng, D.; Wu, J. Org. Lett. 2011, 13, 848.
Miura, S.; Izuta, S. Curr. Drug Targets 2004, 5, 191.
Kolb, H. C.; Sharpless, K. B. Drug Discovery Today 2003, 8, 1128.
Pokhodylo, N.; Shyyka, O.; Matiychuk, V. Sci. Pharm. 2013, 81, 663.
Maurya, S. K.; Gollapalli, D. R.; Kirubakaran, S.; Zhang, M.; Johnson, C. R.; Benjamin, N. N.; Hedstrom, L.; Cuny, G. D. J. Med. Chem. 2009, 52, 4623.
Wen, Y.; Zhang, Z.; Liu, N.-N.; Andrei, G.; Snoeck, R.; Xiang, Y.-H.; Schols, D.; Chen, X.; Zhang, Z.-Y.; Zhang, Q.-S.; Wu, Q.-P. Med. Chem. 2017, 13, 453.
Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057.
Gribanov, P. S.; Topchiy, M. A.; Karsakova, I. V.; Chesnokov, G. A.; Smirnov, A. Yu.; Minaeva, L. I.; Asachenko, A. F.; Nechaev, M. S. Eur. J. Org. Chem. 2017, 5225.
Gribanov, P. S.; Chesnokov, G. A.; Topchiy, M. A.; Asachenko, A. F.; Nechaev, M. S. Org. Biomol. Chem. 2017, 15, 9575.
Afanas'ev, O. I.; Tsyplenkova, O. A.; Seliverstov, M. Y.; Sosonyuk, S. E.; Proskurnina, M. V.; Zefirov, N. S. Russ. Chem. Bull., Int. Ed. 2015, 64, 1470. [Изв. АН, Cер. хим. 2015, 1470.]
Pérez, J. M.; Crosbie, P.; Lal, S.; Díez-González, S. ChemCatChem 2016, 8, 2222.
McIntosh, M L.; Johnston, R. C.; Pattawong, O.; Ashburn, B. O.; Naffziger, M. R.; Cheong, P. H.; Carter, R. G. J. Org. Chem. 2012, 77, 1101.
Akimova, G. S.; Chistokletov, V. N.; Petrov, А. А. Zh. Org. Khim. 1965, 1, 2077.
Akimova, G. S.; Chistokletov, V. N.; Petrov, А. А. Zh. Org. Khim. 1968, 4, 389.
Krasiński, A.; Fokin, V. V.; Sharpless, K. B. Org. Lett. 2004, 6, 1237.
Kwok, S. W.; Fotsing, J. R.; Fraser, R. J.; Rodionov, V. O.; Fokin, V. V. Org. Lett. 2010, 12, 4217.
Maddani, M. R.; Moorthy, S. K.; Prabhu, K. R. Tetrahedron 2010, 66, 329.
Kawamoto, H.; Ito, S.; Satoh, A.; Nagatomi, Y.; Hirata, Y.; Kimura, T.; Suzuki, G.; Sato, A.; Ohta, H. Eur. Patent 1764362.
The study was carried out with the financial support of the Russian Foundation for Basic Research in the framework of the scientific project No. 18-33-00075 mole_a.
The work was carried out using the equipment of the Collective Use Center “Bioorganic” of the Russian Academy of Sciences, supported by the Russian Ministry of Education and Science (agreement identifier RFMEFI62117X0018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supporting information file containing 1H and 13C NMR spectra of the synthesized compounds is available at the journal website at http://link.springer.com/journal/10593.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(7), 755–757
Electronic supplementary material
ESM 1
(PDF 1309 kb)
Rights and permissions
About this article
Cite this article
Karsakova, I.V., Smirnov, A.Y. & Baranov, M.S. An effective method for the synthesis of 1,5-disubstituted 4-halo-1H-1,2,3-triazoles from magnesium acetylides. Chem Heterocycl Comp 54, 755–757 (2018). https://doi.org/10.1007/s10593-018-2343-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2343-6