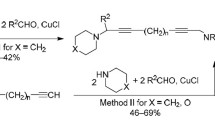
An effective method has been developed for the synthesis of S,N- and S,N,O-containing cyclophanes by cyclothiomethylation reaction of primary amines with formaldehyde and aromatic dithiols. Benzene-1,2-dithiols were used in [1+2+1] cyclocondensation reaction that gave N-substituted 1,5,3-benzodithiazepines, while benzene-1,4-dithiol and 4,4'-dimercaptodiphenyl oxide participated in a [3+6+3] cyclocondensation reaction leading to cyclophanes.



Similar content being viewed by others
References
(a) Multicomponent Reactions: Concepts and Applications for Design and Synthesis; Herrera, R. P.; Marqués-López, E., Eds.; Wiley-VCH Verlag, 2015, 532 p. (b) Multicomponent Reactions in Organic Synthesis; Zhu, J.; Wang, Q.; Wang, M.-X., Eds.; Wiley-VCH Verlag, 2015, 512 p. (c) Dasari, R.; Kornienko, A. Chem. Heterocycl. Compd. 2014, 50, 139. [Khim. Geterotsikl. Soedin. 2014, 160.] (d) Dömling, A. Chem. Rev. 2006, 106, 17.
(a) Akhmetova, V. R.; Nadyrgulova, G. R.; Niatshina, Z. T.; Dzhemilev, U. M. Chem. Heterocycl. Compd. 2009, 45, 1155. [Khim. Geterotsikl. Soedin. 2009, 1443.] (b) Dotsenko, V. V.; Frolov, K. A.; Krivokolysko, S. G. Chem. Heterocycl. Compd. 2015, 51, 109. [Khim. Geterotsikl. Soedin. 2015, 51, 109.] (c) Li, Y.; Yin, G.; Guo, H.; Zhou, B.; Wu, A. Synthesis 2006, 2897. (d) Akhmetova, V. R.; Akhmadiev, N. S.; Ibragimov, A. G. Russ. Chem. Bull., Int. Ed. 2016, 65, 1653. [Izv. Akad. Nauk, Ser. Khim. 2016, 1653.]
Akhmetova, V. R.; Nadyrgulova, G. R.; Khafizova, S. R.; Tyumkina, T. V.; Yakovenko, A. A.; Antipin, M. Yu.; Khalilov, L. M.; Kunakova, R. V.; Dzhemilev, U. M. Russ. Chem. Bull., Int. Ed. 2006, 55, 312. [Izv. Akad. Nauk, Ser. Khim. 2006, 305.]
Pang, T.; Yang, Q.; Gao, M.; Wang, M.; Wu, A. Synlett 2011, 3046.
Hiraoka M. Crown Compounds: Their Characteristics and Applications [Russian translation]; Mir: Moscow, 1986, p. 39.
(а) Sujatha, S.; Balasubramanian, S.; Varghese, B. Polyhedron 2009, 28, 3723. (b) Borisova, N. E.; Reshetova, M. D.; Ustynyuk, Yu. A. Russ. Chem. Rev. 2007, 76, 785. [Usp. Khim. 2007, 76, 843.] (c) Constable, E. C. Metals and Ligand Reactivity: an Introduction to the Organic Chemistry of Metal Complexes; Wiley-VCH: Weinheim, 2005, p. 135.
(a) Rivera, A.; Quevedo, R. Tetrahedron Lett. 2004, 45, 8335. (b) Makhmudiyarova, N. N.; Khatmullina, G. M.; Meshcheryakova, E. S.; Khalilov, L. M.; Ibragimov, A. G.; Dzhemilev, U. M. ARKIVOC 2016, (iii), 48. (c) Makhmudiyarova, N. N.; Kiyamutdinova, G. M.; Meshcheryakova, E. S.; Ibragimov, A. G.; Dzhemilev, U. M. Russ. J. Org. Chem. 2016, 52, 1419. [Zh. Org. Khim. 2016, 52, 1430.]
(a) Aime, S.; Cavallotti, C.; Gianolio, E.; Giovenzana, G. B.; Palmisano, G.; Sisti, M. Org. Lett. 2004, 6, 1201. (b) Khabibullina, G. R.; Fedotova, E. S.; Meshcheryakovа, Е. S.; Buslaeva, Т. М.; Аkhmetova, V. R.; Ibragimov, А. G. Chem. Heterocycl. Compd. 2016, 52, 840. [Khim. Geterotsikl. Soedin. 2016, 52, 840.] (с) Fattakhov, S. G.; Solov'eva, S. E.; Efremov, Yu. Ya.; Rizvanov, I. Kh.; Reznik, V. S. Russ. J. Gen. Chem. 2001, 71, 469. [Zh. Obshch. Khim. 2001, 71, 506.]
(a) Mani, G.; Guchhait, T.; Kumar, R.; Kumar, S. Org. Lett. 2010, 12, 3910. (b) Cai, Q.; Yang, Q.-W.; Zhang, J.-M. Chin. J. Struct. Chem. 2014, 33, 785. (c) Khairullina, R. R.; Akmanov, B. F.; Tyumkina, T. V.; Talipova, R. R.; Ibragimov, A. G.; Dzhemilev, U. M. Macroheterocycles 2015, 8(1), 89.
Khabibullina, G. R.; Akhmetova, V. R.; Abdullin, M. F.; Tyumkina, T. V.; Khalilov, L. M.; Ibragimov, A. G.; Dzhemilev, U. M. Tetrahedron 2014, 70, 3502.
Mani, G.; Jana, D.; Kumar, R.; Ghorai, D. Org. Lett. 2010, 12, 3212.
Suksai, C.; Tuntulani, T. Chem. Soc. Rev. 2003, 32, 192.
Minkin, V. I. Russ. Chem. Bull., Int. Ed. 2008, 57, 687. [Izv. Akad. Nauk, Ser. Khim. 2008, 673.]
Kawanishi, N.; Sugimoto, T.; Shibata, J.; Nakamura, K.; Masutani, K.; Ikuta, M.; Hirai, H. Bioorg. Med. Chem. Lett. 2006, 16, 5122.
(a) Dmitrieva, S. N.; Sidorenko, N. I.; Kurchavov, N. A.; Vedernikov, A. I.; Freidzon, A. Ya.; Kuz'mina, L. G.; Buryak, A. K.; Buslaeva, T. M.; Bagatur'yants, A. A.; Strelenko, Yu. A.; Howard, J. A. K.; Gromov, S. P. Inorg. Chem. 2011, 50, 7500. (b) Khabibullina, G. R.; Buslaeva, Т. M.; Fedotova, Е. S.; Аkhmetova, V. R.; Ibragimov, А. G. Russ. J. Gen. Chem. 2017, 87, 963. [Zh. Obshch. Khim. 2017, 87, 772.]
Khabibullina, G. R.; Akhmetova, V. R.; Fedotova, E. S.; Nigmatullin, V. R.; Nigmatullin, R. G.; Ibragimov, A. G. Petroleum Chemistry 2016, 56, 879. [Neftekhimiya 2016, 56, 662.]
Klimovitskii, E. N.; Litvinov, I. A.; Kataeva, O. N.; Strel’nik, D. Yu.; Sergeeva, G. N. J. Mol. Struct. 1989, 197, 1.
Starosotnikov, A. M.; Nikol’skiy, V. V.; Borodulya, A. N.; Kachala, V. V.; Bastrakov, M. A.; Solkan, V. N.; Shevelev, S. A. Asian J. Org. Chem. 2016, 5, 685.
Tyumkina, T. V.; Khalilov, L. M.; Nadyrgulova, G. R.; Akhmetova, V. R.; Yakovenko, A.; Antipin, M. Yu.; Dzhemilev, U. M. Butlerovskie Soobscheniya 2006, 9(5), 10.
Schöniger, W. Mikrochim. Acta 1956, 44, 869.
CrysAlis PRO; Agilent Technologies: Yarnton, 2012.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Acknowledgement
This work received financial support from the Grants Council of the President of the Russian Federation (grant NSh-5240.2018.3) and funding from the Project part of the State assignment (AAAA-A17-117012610060-7 and AAAAA17-117011910027-0)
The structural characterization of the compounds was performed at the Collective Use Center “Agidel” of the Institute of Petrochemistry and Catalysis of the Russian Academy of Sciences. Mass spectra of compounds 3с,d, 4e,f, 6, and 8a,b were recorded at the Collective Use Center “Chemistry” of the Ufa Institute of Chemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(7), 744–750
Electronic supplementary material
ESM 1
(PDF 927 kb)
Rights and permissions
About this article
Cite this article
Khabibullina, G.R., Fedotova, E.S., Tyumkina, T.V. et al. Cyclothiomethylation of primary amines with formaldehyde and aromatic dithiols – an effective method for the synthesis of cyclophanes. Chem Heterocycl Comp 54, 744–750 (2018). https://doi.org/10.1007/s10593-018-2341-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2341-8