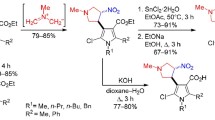
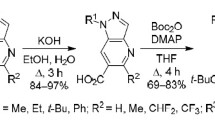
Ethyl 2-oxo-3-(4-oxo-4Н-chromen-2-yl)propanoates reacted with S-methylisothiosemicarbazide hydroiodide at the ethoxalyl group, forming 3-methylsulfanyl-6-[(4-oxo-4Н-chromen-2-yl)methyl]-1,2,4-triazin-5(2H)-ones upon refluxing in pyridine (40–50% yields) or 6-[(4-oxo-4Н-chromen-2-yl)methyl]-1,2,4-triazine-3,5(2Н,4Н)-diones when refluxed in ethanol (37–74% yields).
Similar content being viewed by others
References
Jones, W. D. J. Chem. Soc., Perkin Trans. 1 1981, 344.
Ibrahim, S. S.; El-Shaaer, H. M.; Hassan, A. Phosphorus, Sulfur Silicon Relat. Elem. 2002, 177, 151.
(a) Eftekhari-Sis, B.; Zirak, M. Chem. Rev. 2015, 115, 151. (b) Sakhno, Y. I.; Murlykina, M. V.; Morozova, A. D.; Kozyryev, A. V.; Chebanov, V. A. Fr.–Ukr. J. Chem. 2015, 3, 1.
Safrygin, А. V.; Vetyugova, D. А.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2016, 52, 1035. [Khim. Geterotsikl. Soedin. 2016, 52, 1035.]
Sosnovskikh, V. Ya.; Vetyugova, D. A.; Safrygin, A. V.; Eltsov, O. S.; Slepukhin, P. A. Mendeleev Commun. 2018, 28, 434.
(a) Chang, P. K. J. Org. Chem. 1958, 23, 1951. (b) Andreichikov, Yu. S.; Kol'tsova, S. E.; Zhikina, I. А.; Nekrasov, D. D. Russ. J. Org. Chem. 1999, 35, 1538. [Zh. Org. Khim. 1999, 35, 1567.]
(a) Hajpál, I.; Berényi, E. J. Heterocycl. Chem. 1982, 19, 309. (b) Aliev, Z. G.; Atovmyan, L. O.; Andreichikov, Yu. S.; Kol'tsova, S. E.; Nekrasov, D. D. Russ. Chem. Bull. 1998, 47, 682. [Izv. Akad. Nauk, Ser. Khim. 1998, 47, 704.] (c) Ibrahim, Y. A.; Abdel-Hady, S. A. L.; Badawy, M. A.; Ghazala, M. A. H. J. Heterocycl. Chem. 1982, 19, 913. c Ibrahim, Y. A.; Al-Saleh, B.; Mahmoud, A. A. A. Tetrahedron 2003, 59, 8489.
(a) Ibrahim, M. A.; Ali, T. E.; Alnamer, Y. A.; Gabr, Y. A. ARKIVOC 2010, (i), 98. (b) Ghosh, C. K. J. Heterocycl. Chem. 2006, 43, 813.
This work received financial support from the Russian Foundation for Basic Research (grant 17-03-00340).
Analytical studies were performed at the Collective Access Center “Spectroscopy and analysis of organic compounds” of the Institute of Organic Synthesis, Ural Branch of the Russian Academy of Sciences and at the Laboratory of Complex Investigations and Expert Evaluation of Organic Materials of the Ural Federal University named after the first President of Russia B. N. Yeltsin.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(7), 696–699
Rights and permissions
About this article
Cite this article
Vetyugova, D.A., Nashtatik, N.S., Safrygin, A.V. et al. The reaction of ethyl 2-oxo-3-(4-oxo-4Н-chromen-2-yl)propanoates with S-methylisothiosemicarbazide hydroiodide. Chem Heterocycl Comp 54, 696–699 (2018). https://doi.org/10.1007/s10593-018-2334-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2334-7