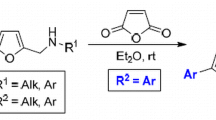
The size of nitrogen heterocycle in N-allyl- and N-propargyl-α-furyl lactams, as well as the nature of the unsaturated substituent linked to the nitrogen atom affected the possibility of thermal intramolecular [4+2] cycloaddition between multiple bond and the furan ring. N-Allyl-γ-(α-furyl)butyrolactam was shown to be unreactive at temperatures from 140 to 230°С. Substituted δ-valero- and ε-caprolactams underwent partial Diels–Alder cyclization, forming tautomeric mixtures that contained both the initial open-chain form and the cyclic form (diastereomeric 3a,6-epoxyisoindoles fused with an aza ring) in ratios between 19:81 and 55:45. N-Propargyl-α-furyl lactams did not participate in thermal IMDAF reaction regardless of the ring size and the temperature of the synthesis.
Similar content being viewed by others
References
(a) Reiser, O.; Seitz, M. Curr. Opin. Chem. Biol. 2005, 9, 285. (b) Gil, S.; Parra, M.; Rodriguez, P.; Segura, J. Mini-Rev. Org. Chem. 2009, 6, 345. (c) Wei, L.; Malhotra, S. V. Curr. Med. Chem. 2010, 17, 234. (d) Fagnoni, M.; Bonassi, F.; Palmieri, A.; Protti, S.; Ravelli, D.; Ballini, R. Adv. Synth. Catal. 2014, 356, 753. (e) Natural Lactones and Lactams: Synthesis, Occurrence and Biological Activity; Janecki, T., Ed.; Wiley Publications, 2014.
(a) Parvatkar, P. T.; Kadam, H. K.; Tilve, S. G. Tetrahedron 2014, 70, 2857. (b) Padwa, A.; Flick, A. C. Adv. Heterocycl. Chem. 2013, 110, 1. (c) Juhl, M.; Tanner, D. Chem. Soc. Rev. 2009, 38, 2983. d Zubkov, F. I.; Nikitina, E. V.; Varlamov, A. V. Russ. Chem. Rev. 2005, 74, 639. [Usp. Khim. 2005, 74, 707.] (d) Vogel, P.; Cossy, J.; Plumet, J.; Arjona, O. Tetrahedron 1999, 55, 13521. (e) Kappe, C. O.; Murphree, S. S.; Albert, P. Tetrahedron 1997, 53, 14179.
(a) Alaşalvar, C.; Demircan, A.; Koşar, B.; Pekacar, A. I.; Büyükgüngör, O. J. Mol. Struct. 2016, 1123, 213. (b) Demircan, A.; Kandemir, M. K.; Colak, M.; Karaarslan, M. Synthesis 2016, 2873. (c) Mason, K. M.; Meyers, M. S.; Fox, A. M.; Luesse, S. B. Beilstein J. Org. Chem. 2016, 12, 2032. (d) Zaytsev, V. P.; Zubkov, F. I.; Nadirova, M. A.; Mertsalov, D. F.; Nikitina, E. V.; Novikov, R. A.; Varlamov, A. V. Chem. Heterocycl. Compd. 2016, 52, 736. [Khim. Geterotsikl. Soedin. 2016, 52, 736.] (e) Chen, C.-H.; Yellol, G. S.; Tsai, C.-H.; Dalvi, P. B.; Sun, C.-M. J. Org. Chem. 2013, 78, 9738. e Rae, R. L.; Zurek, J. M.; Paterson, M. J.; Bebbington, M. W. P. Org. Biomol. Chem. 2013, 11, 7946. f Zubkov, F. I.; Nikitina, E. V.; Zaytsev, V. P.; Khrustalev, V. N.; Novikov, R. A; Borisov, R. S.; Varlamov, A. V. Chem. Heterocycl. Compd. 2012, 48, 785. [Khim. Geterotsikl. Soedin. 2012, 844.] (h) Claeys, D. D.; Stevens, C. V.; Roman, B. I.; Van De Caveye, P.; Waroquier, M.; Van Speybroeck, V. Org. Biomol. Chem. 2010, 8, 3644. g Claeys, D. D.; Moonen, K.; Roman, B. I.; Nemykin, V. N.; Zhdankin, V. V.; Waroquier, M.; Van Speybroeck, V.; Stevens, C. V. J. Org. Chem. 2008, 73, 7921. h Varlamov, A. V.; Boltukhina, E. V.; Zubkov, F. I.; Nikitina, E. V.; Turchin, K. F. J. Heterocycl. Chem. 2006, 43, 1479. i Namboothiri, I. N. N.; Ganesh, M.; Mobin, S. M.; Cojocaru, M. J. Org. Chem. 2005, 70, 2235. j Varlamov, A. V.; Boltukhina, E. V.; Zubkov, F. I.; Nikitina, E. V.; Turchin, K. F. J. Heterocycl. Chem. 2006, 43, 1479.
(a) Nyberg, K.; Servin, R. Acta Chem. Scand. 1976, B30, 640. (b) Warning, K.; Mitzlaff, M. Tetrahedron Lett. 1979, 20, 1563. (c) Shono, T.; Matsumura, Y.; Tsubata, K.; Sugihara, Y.; Yamane, S.; Kanazawa, T.; Aoki, T. J. Am. Chem. Soc. 1982, 104, 6697. (d) Shono, T.; Matsumura, Y.; Tsubata, K. Org. Synth. Coll. Vol. 1990, 7, 307.
(a) Edwards, O. E.; Greaves, A. M.; Sy, W. W. Can. J. Chem. 1988, 66, 1163. (b) Ben-Ishai, D.; Sataty, I.; Bernstein, Z. Tetrahedron 1976, 32, 1571. (c) Shono, T.; Matsumura, Y.; Tsubata, K.; Takata, J. Chem. Lett. 1981, 1121.
Vasse, J.; Levacher, V.; Bourguignon, J.; Dupas, G. Tetrahedron 2003, 59, 4911.
Zubkov, F. I.; Golubev, V. D.; Zaytsev, V. P.; Bakhanovich, O. V.; Nikitina, E. V.; Khrustalev, V. N.; Aysin, R. R.; Timofeeva, T. V.; Novikov, R. A.; Varlamov, A. V. Chem. Heterocycl. Compd. 2016, 52, 225. [Khim. Geterotsikl. Soedin. 2016, 52, 225.]
(a) Lu, Q.; Huang, X.; Song, G.; Sun, C.-M.; Jasinski, J. P.; Keeley, A. C.; Zhang, W. ACS Comb. Sci. 2013, 15, 350. (b) Ghelfi, F.; Parsons, A. F.; Tommasini, D.; Mucci, A. Eur. J. Org. Chem. 2001, 1845. (c) Zubkov, F. I.; Nikitina, E. V.; Galeev, T. R.; Zaytsev, V. P.; Khrustalev, V. N.; Novikov, R. A.; Orlova, D. N.; Varlamov, A. V. Tetrahedron 2014, 70, 1659.
(a) Spare, L. K.; Falsetta, P.; Gilbert, J.; Harman, D. G.; Baker, M. A.; Li, F.; McCluskey, A.; Clegg, J. K.; Sakoff, J. A.; Aldrich-Wright, J. R.; Gordon, C. P. ChemMedChem 2017, 12, 130. (b) Gordon, C. P.; Young, K. A.; Robertson, M. J.; Hill, T. A.; McCluskey, A. Tetrahedron 2011, 67, 554. (c) Gordon, C. P.; Byrne, N.; McCluskey, A. Green Chem. 2010, 12, 1000.
Tagmazyan, K. Ts.; Mkrtchyan, R. S.; Babayan, A. T. Zh. Org. Khim. 1974, 10, 1642.
(a) Shono, T.; Matsumura, Y.; Uchida, K.; Kobayashi, H. J. Org. Chem. 1985, 50, 3243. (b) Pereira, E. R.; Sancelme, M.; Towa, J.-J.; Prudhomme, M.; Martre, A.-M.; Mousset, G.; Rapp, M. J. Antibiotics 1996, 49, 380. (c) Nishitani, T.; Horikawa, H.; Iwasaki, T.; Matsumoto, K.; Inoue, I.; Miyoshi, M. J. Org. Chem. 1982, 47, 1706. (d) Shono, T.; Matsumura, Y.; Kanazawa, T. Tetrahedron Lett. 1983, 12, 1259.
Ciufolini, M. A.; Wood, C. Y. Tetrahedron Lett. 1986, 27, 5085.
Synthesis of the starting compounds 2 and 3 was supported by the Russian Foundation for Basic Research (grant No. 17-53-45016). The synthesis and NMR spectroscopy of adducts 4 were performed with financial support from the RUDN University program ''5-100''.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(4), 451–457
Electronic supplementary material
ESM 1
(PDF 6197 kb)
Rights and permissions
About this article
Cite this article
Poplevin, D.S., Nikitina, E.V., Zaytsev, V.P. et al. Intramolecular [4+2] cycloaddition in N-allyl- and N-propargyl-α-furyl lactams. Chem Heterocycl Comp 54, 451–457 (2018). https://doi.org/10.1007/s10593-018-2290-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2290-2