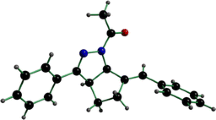
Dimethyl 3-(2-bromophenyl)dibenzo[b,f]pyrrolo[1,2-d][1, 4]oxazepine-1,2-dicarboxylate and its 3,13b-dihydro and 1,2,3,13b-tetrahydro analogs undergo intramolecular cyclization under free radical conditions with the formation of dimethyl 7-oxa-2a1-azabenzo[b]-cyclopenta[pq]pleiadene-1,2-dicarboxylate, the unique representative of pyrrole-fused dibenzo[b,f][1, 4]oxazepines, consisting of six fused rings. This nonplanar oxazapolyheterocycle shows a strong blue emission in dichloromethane.



Similar content being viewed by others
References
(a) Tomashenko, O. A.; Khlebnikov, A. F.; Mosiagin, I. P.; Novikov, M. S.; Grachova, E. V.; Shakirova, J. R.; Tunik, S. P. RCS Adv. 2015, 5, 94551. (b) Tomashenko, O. A.; Novikov, M. S.; Khlebnikov, A. F. J. Org. Chem. 2017, 82, 616.
(a) Khlebnikov, A. F.; Golovkina, M. V.; Novikov, M. S.; Yufit, D. S. Org. Lett. 2012, 14, 3768. (b) Khlebnikov, A. F.; Tomashenko, O. A.; Funt, L. D.; Novikov, M. S. Org. Biomol. Chem. 2014, 12, 6598.
Khlebnikov, A. F.; Novikov, M. S.; Petrovskii, P. P.; Konev, A. S.; Yufit, D. S.; Selivanov, S. I.; Frauendorf, H. J. Org. Chem. 2010, 75, 5211.
(a) Gijsen, H. J. M.; Berthelot, D.; Zaja, M.; Brône, B.; Geuens, I.; Mercken, M. J. Med. Chem. 2010, 53, 7011. (b) Binaschi, M.; Boldetti, A.; Gianni, M.; Maggi, C. A.; Gensini, M.; Bigioni, M.; Parlani, M.; Giolitti, A.; Fratelli, M.; Valli, C.; Terao, M.; Garattini, E. ACS Med. Chem. Lett. 2010, 1, 411. (c) Deraeve, C.; Guo, Z.; Bon, R. S.; Blankenfeldt, W.; Di Lucrezia, R.; Wolf, A.; Menninger, S.; Stigter, E. A.; Wetzel, S.; Choidas, A.; Alexandrov, K.; Waldmann, H.; Goody, R. S.; Wu, Y.-W. J. Am. Chem. Soc. 2012, 134, 7384. (d) Chen, C.-L.; Lee, C.-C.; Liu, F.-L.; Chen, T.-C.; Ahmed Ali, A. A.; Chang, D.-M.; Huang, H.-S. Eur. J. Med. Chem. 2016, 117, 70. (e) Yu, W.; Coburn, C. A.; Yang, D.-Y.; Meinke, P. T.; Wong, M.; Rosenblum, S. B.; Chen, K. X.; Njoroge, G. F.; Chen, L.; Dwyer, M. P.; Jiang, Y.; Nair, A. G.; Selyutin, O.; Tong, L.; Zeng, Q.; Zhong, B.; Ji, T.; Hu, B.; Agrawal, S.; Xia, E.; Zhai, Y.; Liu, R.; Kong, R.; Ingravallo, P.; Asante-Appiah, E.; Nomeir, A.; Fells, J.; Kozlowski, J. A. Bioorg. Med. Chem. Lett. 2016, 26, 3158.
(a) Hu, F.; Liu, H.; Jia, J.; Ma, C. Org. Biomol. Chem. 2016, 14, 11076. (b) Sang, P.; Yu, M.; Tu, H.; Zou, J.; Zhang, Y. Chem. Commun. 2013, 701.
(a) Grzybowski, M.; Glodkowska-Mrowka, E.; Clermont, G.; Blanchard-Desce, M.; Gryko, D. T. Chem. Heterocycl. Compd. 2017, 53, 72. [Khim. Geterotsikl. Soedin. 2017, 53, 72.] (b) Santra, M.; Jun, Y. W.; Bae, J.; Sarkar, S.; Choi, W.; Gryko, D. T.; Ahn, K. H. Asian J. Org. Chem. 2017, 6, 278. (c) Ge, Y.; Liu, A.; Dong, J.; Duan, G.; Cao, X.; Li, F. Sens. Actuators, B 2017, 247, 46. (d) Łukasiewicz, Ł. G.; Deperasińska, I.; Poronik, Y. M.; Jun, Y. W.; Banasiewicz, M.; Kozankiewicz, B.; Ahn, K. H.; Gryko, D. T. Chem.–Asian J. 2016, 11, 1718. (e) Yu, C.; Wu, Q.; Wang, J.; Wei, Y.; Hao, E.; Jiao, L. J. Org. Chem. 2016, 81, 3761. (f) Sareen, D.; Lee, J. H.; Hwang, H.; Yoo, S.; Lee, C.-H. Chem. Commun. 2016, 52, 5852. (g) Koshel, E. I.; Tomashenko, O. A.; Khlebnikov, A. F.; Gaginskaya, E. R.; Saifitdinova, A. F.; Tunik, S. P. Chromosome Res. 2016, 24 (Suppl 1), 29. DOI: 10.1007/s10577-016-9532-x
Togo, H. Advanced Free Radical Reactions for Organic Synthesis; Elsevier: Amsterdam, 2004, p. 1.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09. Revision D.01; Gaussian, Inc.: Wallingford, 2009.
Khlebnikov, A. F.; Novikov, M. S.; Petrovskii, P. P.; Magull, J.; Ringe, A. Org. Lett. 2009, 11, 979.
X-Area & X-RED32 Software; Stoe & Cie GmbH: Darmstadt, 2016.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
Spek, A. L. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2009, D65, 148.
We gratefully acknowledge the financial support of the Russian Science Foundation (grant No. 16-13-10036). This research was carried out using resources of the Center for Magnetic Resonance, the Computer Center, and the Center for Chemical Analysis and Materials, the Center for Optical and Laser Materials Research of Saint Petersburg State University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(8), 909–912
Rights and permissions
About this article
Cite this article
Petrovskii, P.P., Tomashenko, O.A., Novikov, M.S. et al. Synthesis, crystal structure, and photophysical properties of dimethyl 7-oxa-2a1-azabenzo[b]cyclopenta[pq]pleiadene-1,2-dicarboxylate – novel fused oxazapolycyclic skeleton. Chem Heterocycl Comp 53, 909–912 (2017). https://doi.org/10.1007/s10593-017-2144-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-2144-3