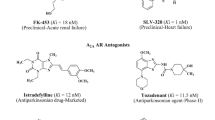
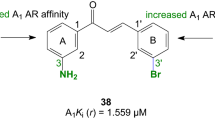
In the context of a program to identify selective adenosine A2B receptor antagonists, we have obtained a focused library of 4-substituted 3,4-dihydropyrimidin-2(1H)-ones and its affinity for the four human adenosine receptor subtypes was determined. The synthesis was accomplished by using an experimentally simple and efficient Biginelli approach. The biological evaluation of the library revealed that all the documented derivatives exhibit low or negligible affinity for the A2B receptor, thus highlighting the critical importance of the substituent at position 4 of the 3,4-dihydropyrimidin-2(1H)-one chemotype.


Similar content being viewed by others
References
Fredholm, B. B.; Arslan, G.; Halldner, L.; Kull, B.; Shulte, G.; Wasserman, W. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000, 362, 299.
Lennarz, W. J., Lane, M. D. Biol. Chem. 2004, 1, 34.
Fredholm, B. B.; Ijzerman, A. P.; Jacobson, K. A.; Linden, J.; Muller, C. E. Pharmacol. Rev. 2011, 63, 1.
Moro, S.; Deflorian, F.; Bacilieri, M.; Spalluto, G. Curr. Pharm. Des. 2006, 12, 2175.
Fredholm, B. B.; Irenius, E.; Kull, B.; Schulte, G. Biochem. Pharmacol. 2001, 61, 443.
Adenosine Receptors in Health and Disease. Handbook of Experimental Pharmacology; Wilson, C. N.; Mustafa, S. J.,Eds.; Springer-Verlag: Berlin, Heidelberg, 2009, Vol. 193.
Baraldi, P. G.; Tabrizi, M. A.; Gessi, S.; Borea, P. A. Chem. Rev. 2008, 108, 238.
Jacobson, K. A.; Gao, Z. G. Nat. Rev. Drug Discovery 2006, 5, 247.
Pierce, K. D.; Furlong, T. J.; Selbie, L. A.; Shine, J. Biochem. Biophys. Res. Commun. 1992, 187, 86.
Fredholm, B. B.; Ijzerman, A. P.; Jacobson, K. A.; Klotz, K. N.; Linden, J. Pharmacol. Rev. 2001, 53, 527.
Aherne, C. M.; Kewley, E. M.; Eltzschig, H. K. Biochim. Biophys. Acta 2011, 1808, 1329.
Cacciari, B.; Pastorin, G.; Bolcato, C.; Spalluto, G.; Bacilieri, M.; Moro, S. Mini Rev. Med. Chem. 2005, 5, 1053.
Crespo, A.; El Maatougui, A.; Biagini, P.; Azuaje, J.; Coelho, A.; Brea, J.; Loza, M. I.; Cadavid, M. I.; García-Mera, X. Gutiérrez-de-Terán, H.; Sotelo, E. ACS Med. Chem. Lett. 2013, 4, 1031.
Sun, Q.; Wang, Y.; Ge, Z.; Cheng, T.; Li, R. Synthesis 2004, 1047.
Kappe, C. O. Eur. J. Med. Chem. 2000, 35, 1043.
Dömling, A. Chem. Rev. 2006, 106, 17.
Al Maatougui, A.; Azuaje, J.; González-Gómez, M.; Miguez, G.; Crespo, A.; Carbajales, C.; Escalante, L.; García-Mera, X.; Gutiérrez-de-Terán, H.; Sotelo, E. J. Med. Chem. 2016, 59, 1967.
Xu, F.; Wang, J. J.; Tian, Y. P. Synth. Commun. 2008, 38, 1299.
Gholap, A. R.; Venkatesan, K.; Daniel, T.; Lahoti, R. J.; Srinivasan, K. V. Green Chem. 2004, 6, 147.
Song, Q.; An, X.; Che, F.; Shen, T. J. Heterocycl. Chem. 2015, 52, 1496.
Cepanec, I.; Litvic, M.; Bartolincic, A.; Lovric, M. Tetrahedron 2005, 61, 4275.
Hajipour, A. R.; Khazdooz, L.; Zarei, A. Synth. Commun. 2011, 41, 2200.
Chitra, S.; Devanathan, D.; Pandiarajan, K. Eur. J. Med. Chem. 2010, 45, 367.
Niknam, K.; Zolfigol, M. A.; Hossieninejad, Z.; Daneshvar, N. Chin. J. Catal. 2007, 28, 591.
Acknowledgements
This work was financially supported by the Concellería de Cultura, Educación e Ordenación Universitaria of the Galician Government (grant GPC2014/03), Centro Singular de Investigación de Galicia Accreditation 2016–2019 (ED431G/09), and the European Regional Development Fund (ERDF). Our laboratories are part of the European COST Actions CM1207 (GLISTEN) and CM15135 (MuTaLig).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(3), 316–322
Rights and permissions
About this article
Cite this article
Crespo, A., El Maatougui, A., Azuaje, J. et al. Exploring the influence of the substituent at position 4 in a series of 3,4-dihydropyrimidin-2(1H)-one A2B adenosine receptor antagonists. Chem Heterocycl Comp 53, 316–321 (2017). https://doi.org/10.1007/s10593-017-2054-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-2054-4