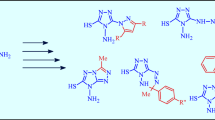
A reaction of 2-cyanoacetamide with benzylideneacetone in DMSO containing potassium tert-butoxide was used to synthesize 3-cyano-6-methyl-4-phenylpyridin-2(1H)-one, which was converted by acidic hydrolysis to 6-methyl-2-oxo-4-phenyl-1,2-dihydropyridine-3-carboxamide. A Hofmann reaction of this compound in the presence of sodium hypobromite led to 3-amino-5-bromo-6-methyl-4-phenylpyridin-2(1H)-one, while its treatment with calcium hypochlorite produced 5-methyl-7-phenyloxazolo[5,4-b]pyridin-2(1H)-one. The latter compound was converted by heating with alkali to 3-amino-6-methyl-4-phenylpyridin-2(1H)-one, which gave azomethine in a reaction with benzaldehyde, while N-acylated derivatives were obtained in reactions with acyl halides. The heating of N 1,N 2-bis(6-methyl-2-oxo-4-phenyl-1,2-dihydropyridin-3-yl)oxalylamide in the presence of POCl3 allowed to obtain 5,5'-dimethyl-7,7'-diphenyl-2,2'-bis-(oxazolo[5,4-b]pyridine).






Similar content being viewed by others
References
Kusakabe, K.; Tada, Y.; Iso, Y.; Sakagami, M.; Morioka, Y.; Chomei, N.; Shinonome, S.; Kawamoto, K.; Takenaka, H.; Yasui, K.; Hamana, H.; Hanasaki, K. Bioorg. Med. Chem. 2013, 21, 2045.
Zhang, Y.-M.; Fan, X.; Chakaravarty, D.; Xiang, B.; Scannevin, R. H.; Huang, Z.; Ma, J.; Burke, S. L.; Karnachi, P.; Rhodesa, K. J.; Jackson, P. F. Bioorg. Med. Chem. Lett. 2008, 18, 409.
Crawford, J. J.; Lee, W.; Young, W. B. US Patent 20150011461.
Ward, A.; Brogden, R. N.; Heel, R. C.; Speight, T. M.; Avery, G. S. Drugs 1983, 26, 468.
Verissimo, E.; Berry, N.; Gibbons, P.; Cristiano, M. L. S.; Rosenthal, P. J.; Gut, J.; Ward, S. A.; O'Neill, P. M. Bioorg. Med. Chem. Lett. 2008, 18, 4210.
Ettari, R.; Bonaccorso, C.; Micale, N.; Heindl, C.; Schirmeister, T.; Calabrò, M. L.; Grasso, S.; Zappalà, M. ChemMedChem 2011, 6, 1228.
Yu, M.; Li, P.; Basnet, S. K.C.; Kumarasiri, M.; Diab, S.; Teo, Th.; Albrecht, H.; Wang, Sh. Eur. J. Med. Chem. 2015, 95, 116.
Fisyuk, A. S.; Bundel', Yu. G. Chem. Heterocycl. Compd. 1999, 35, 125. [Khim. Geterotsikl. Soedin. 1999, 147.]
Fissyuk, A. S.; Vorontsova, M. A.; Temnikov, D. V. Tetrahedron Lett. 1996, 37, 5203.
Fisyuk, A. S.; Poendaev, N. V.; Bundel', Y. G. Mendeleev Commun. 1998, 8, 12.
Fisyuk, A. S.; Bogza, Y. P.; Poendaev, N. V.; Goncharov, D. S. Chem. Heterocycl. Compd. 2010, 46, 844. [Khim. Geterotsikl. Soedin. 2010, 1044.]
Goncharov, D. S.; Kostuchenko, A. S.; Fisyuk, A. S. Chem. Heterocycl. Compd. 2009, 45, 793. [Khim. Geterotsikl. Soedin. 2009, 1005.]
Goncharov, D. S.; Garkushenko, A. K.; Savelieva, A. P.; Fisyuk, A. S. ARKIVOC 2015, (v), 176.
Fisyuk, A. S.; Kulakov, I. V.; Goncharov, D. S.; Nikitina, O. S.; Bogza, Y. P.; Shatsauskas, A. L. Chem. Heterocycl. Compd. 2014, 50, 217. [Khim. Geterotsikl. Soedin. 2014, 241.]
Kulakov, I. V.; Matsukevich, M. V.; Shulgau, Z. T.; Sergazy, S.; Seilkhanov, T. M; Puzari, A.; Fisyuk, A. S. Chem. Heterocycl. Compd. 2015, 51, 991. [Khim. Geterotsikl. Soedin. 2015, 51, 991.]
Kulakov, I. V.; Nikitina, O. S.; Fisyuk, A. S.; Goncharov, D. S.; Shul'gau, Z. T.; Gulyaev, A. E. Chem. Heterocycl. Compd. 2014, 50, 670. [Khim. Geterotsikl. Soedin. 2014, 729.]
Jain, R.; Rosshangar, F.; Ciufolini, M. A. Tetrahedron Lett. 1995, 36, 3307.
Gudrinietse, É; Yure, M.; Pastors, P.; Karklinya, A.; Paliatis, É. Chem. Heterocycl. Compd. 1995, 31, 243. [Khim. Geterotsikl. Soedin. 1995, 271.]
Yure, M. V.; Shantare, D. V.; Gurdinietse, É. Yu. Chem. Heterocycl. Compd. 1996, 32, 473. [Khim. Geterotsikl. Soedin. 1996, 542.]
Irlapati, N. R.; Khedkar, N. R.; Jape, R. B.; Nandurdikar, R. Sh.; Shaikh, Z. A. W.; Sinha, N.; Palle, V. P; Kamboj, R. K. WO Patent 2014203217
Huang, Z.; Zhang, Y.; Song, Y. WO Patent 2011085643.
Viaud, M.-C.; Jamoneau, P.; Flouzat, Ch.; Bizot-Espiard, J.-G.; Pfeiffer, B.; Renard, P.; Caignard, D.-H.; Adam, G.; Guillaumet, G. J. Med. Chem. 1995, 38, 1278.
Ockenden, W.; Schofield, K. J. Chem. Soc. 1953, 3914.
Schofield, K.; Theobald, R. S. J. Chem. Soc. 1951, 2992.
Speckamp, W. N.; Hiemstra, H. Tetrahedron, 1985, 41, 4367.
Fisyuk, A. S.; Mukanov, A. Yu.; Novikova, E. Yu. Mendeleev Commun. 2003, 6, 278.
Fisyuk, A. S.; Mukanov, A. Yu. Chem. Heterocycl. Compd. 2003, 39, 277. [Khim. Geterotsikl. Soedin. 2003, 307.]
Fisyuk, A. S.; Mukanov, A. Yu. Russ. J. Org. Chem. 2006, 42, 1269. [Zh. Org. Khim. 2006, 42, 1291.]
Fisuyk, A. S.; Mukanov, A. Y.; Poendaev, N. V. Mol. Diversity 2010, 14, 455.
Fisyuk, A. S. Chem. Heterocycl. Compd. 2012, 48, 548. [Khim. Geterotsikl. Soedin. 2012, 588.]
Bomika, Z. A.; Andaburskaya, M. B.; Pelcher, Yu. É.; Dubur, G. Ya. Chem. Heterocycl. Compd. 1975, 11, 967. [Khim. Geterotsikl. Soedin. 1975, 1108.]
The work was performed with financial support from the Russian Foundation for Basic Research (project 15-53- 45084 IND_a).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(2), 186–191
Rights and permissions
About this article
Cite this article
Shatsauskas, A.L., Abramov, A.A., Saibulina, E.R. et al. Synthesis of 3-amino-6-methyl-4-phenylpyridin-2(1H)-one and its derivatives. Chem Heterocycl Comp 53, 186–191 (2017). https://doi.org/10.1007/s10593-017-2038-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-2038-4