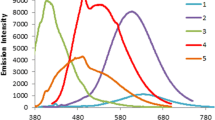
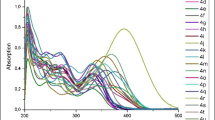
Thiophenes and phenylacetylenes, decorated with 1-methylindol-2-yl, 1,3,2-benzodiazaborol-2-yl, 1,3,2-diazaborol-2-yl, or 1,3,2-diazaborolidinyl groups at one end and dimesitylborolyl or CN substituents at the opposite end of the molecules, were synthesized. Upon UV irradiation, these push-pull systems in THF solution gave rise to bright-blue emission with Stokes shifts ranging from 4100 to 9300 cm–1 and quantum efficiencies up to 0.99. Thereby intramolecular charge transfer took place from the HOMO of the indolyl or borolyl fluorophores to the LUMO, mainly centered on the electron-withdrawing cyano or dimesitylboranyl group.




Similar content being viewed by others
References
(a) Entwistle, C. D.; Marder, T. B. Angew. Chem., Int. Ed. 2002, 41, 2927. (b) Entwistle, C. D.; Marder, T. B. Chem. Mater. 2004, 16, 4574. (c) Yamaguchi, S.; Wakayama, A. Pure Appl. Chem. 2006, 78, 1413. (d) Jäkle, F. Coord. Chem. Rev. 2006, 250, 1107.
(a) Yuan, Z.; Taylor, N. J.; Marder, T. B.; Williams, D. S.; Kurtz, S. K.; Cheng, L.-T. J. Chem. Soc., Chem. Commun. 1990, 1489. (b) Yuan, Z; Taylor, N. J.; Marder, T. B.; Williams, I. D.; Kurtz, S. K.; Cheng, L-T. Organic Materials for Non-Linear Optic II; Hann, R. A.; Bloor, D., Eds.; Royal Society of Chemistry: Cambridge, 1991, p. 190. (c) Lequan, M.; Lequan, R. M.; Chane-Ching, K. J. Mater. Chem. 1991, 1, 997. (d) Lequan, M.; Lequan, R. M.; Chane-Ching, K.; Barzoukas, M.; Fort, A ; Lahoucine, H.; Bravic, G.; Chasseau, J.; Gaultier, J. J Mater. Chem. 1992, 2, 719. (e) Lequan, M.; Lequan, R. M.; Chang-Ching, K.; Callier, A.-C.; Barzoukas, M.; Fort, A. Adv. Mater. Opt. Electron. 1992, 1. 243. (f) Yuan, Z.; Taylor, N. J.; Sun, Y.; Marder, T. B.; Williams, I. D.; Cheng, L.-T. J. Organomet. Chem. 1993, 449, 27. (g) Branger, C.; Lequan, M.; Lequan, R. M.; Barzoukas, M.; Fort, A. J. Mater. Chem. 1996, 6, 555. (h) Yuan, Z.; Taylor, N. J.; Ramachandran, R.; Marder, T. B. Appl. Organomet. Chem. 1996, 10, 305. (i) Yuan, Z.; Collings, J. C.; Taylor, N. J.; Marder, T. B.; Jardin, C.; Halet, J.-F. J. Solid State Chem. 2000, 154, 5. (j) Liu, Y.; Xu, X; Zheng, F.; Cui, Y. Angew. Chem., Int. Ed. 2008, 47, 4538.
Yuan, Z.; Entwistle, C. D.; Collings, J. C.; Albesa-Jové, D.; Batsanov, A. S.; Howard, J. A. K.; Taylor, N. J.; Kaiser, K. M.; Kaufmann, D. E.; Poon, S.-Y.; Wong, W. J.; Jardin, C.; Fathallah, S.; Boucekkine, A.; Halet, J.-F.; Marder, T. B. Chem.–Eur. J. 2006, 12, 2758.
(a) Liu, Z .Q.; Fang, Q.; Wang, D.; Xue, G.; Yu, W. T.; Shao, Z. S.; Jiang, M.-H. Chem. Commun. 2002, 2900. (b) Liu, Z.-Q.; Fang, Q.; Cao, D. X.; Xue, G.; Yu, W. T.; Lei, H. Chem.–Eur. J. 2003, 9, 5074. (c) Cao, D. X.; Liu, Z.-Q.; Fang, Q.; Xu, G.-B.; Xue, G.; Liu, G.-Q.; Yu, W.-T. J. Organomet. Chem. 2004, 689, 2201. (d) Liu, Z.-Q.; Fang, Q.; Cao, D.-X.; Wang, D.; Xu, G.-B. Org. Lett. 2004, 6, 2933. (e) Liu, Z.-Q.; Shi, M.; Li, F.-Y.; Fang, Q.; Chen, Z.-H.; Yi, T.; Huang, C.-H. Org. Lett. 2005, 7, 5481. (f) Charlot, M.; Porrès, L.; Entwistle, C. D.; Beeby, A.; Marder, T. B.; Blanchard-Desce, M. Phys. Chem. Chem. Phys. 2005, 7, 600. (g) Porrès, L.; Charlot, M.; Entwistle, C. D.; Beeby, A.; Marder, T. B.; Blanchard-Desce, M. Proc. SPIE-Int. Soc. Opt. Eng. 2005, 5934, 59340F. (h) Cao, D.-X.; Liu, Z.-Q; Li, G.-Z.; Liu, G.-Q.; Zhang, G.-H. J. Mol. Struct. 2008, 874, 46. (i) Collins, J. C.; Poon, S.-Y.; Le Droumaguet, C.; Charlot, M.; Katan, C.; Pålsson, L.-O.; Beeby, A.; Mosely, J. A.; Kaiser, H. M.; Kaufmann, D.; Wong, W.-Y.; Blanchard-Desce, M.; Marder, T. B. Chem.–Eur. J. 2009, 15, 198. (j) Zhao, S.-B.; Wucher, P.; Mc Cormick, T. M.; Liu, X.-Y.; Wang, S.; Feng, X.-D.; Lu, Z.-H.; Organometallics 2008, 27, 6446.
(a) Noda, T.; Shirota, Y. J. Am. Chem. Soc. 1998, 120, 9714. (b) Noda, T.; Ogawa, H.; Shirota, Y. Adv. Mater. 1999, 11, 283. (c) Noda, T.; Shirota, Y. J. Lumin. 2000, 87–89, 1168. (d) Shirota, Y.; Kinoshita, M.; Noda, T.; Okumoto, K.; Ohara, T. J. Am. Chem. Soc. 2000, 122, 11021. (e) Kinoshita, M.; Fujii, N.; Tsuzuki, T.; Shirota, Y. Synth. Met. 2001, 121, 1571. (f) Doi, H.; Kinoshita, M.; Okumotu, K.; Shirota, Y. Chem. Mater. 2003, 15, 1080. (g) Jia, W.-L.; Bai, D.-R.; Mc Cormick, T.; Liu, Q.-D.; Motala, M.; Wang, R.-Y.; Seward, C.; Tao, Y; Wang, S. Chem.–Eur. J. 2004, 10, 994. (h) Jia, W.-L.; Feng, D.; Bai, D. R.; Lu, Z.-H.; Wang, S.; Vamvounis, G. Chem. Mater. 2005, 17, 164. (i) Mazzeo, M.; Vitale, V.; Della Sala, F.; Anni, M.; Barbarella, G.; Favaretto, L.; Sotgui, G.; Cingolani, R.; Gigli, G. Adv. Mater. 2005, 17, 34. (j) Zhou, G.-J.; Ho, C.-L.; Wong, W.-Y.; Wang, Q.; Ma, D.-G.; Wang, L.-X.; Lin, Z.-Y.; Marder, T. B.; Beeby, A. Adv. Funct. Mater. 2008, 18, 499.
Glogowski, M. E.; Williams, J. L. R. J. Organomet. Chem. 1981, 218, 137.
Schulz, A.; Kaim, W. Chem. Ber. 1989, 122, 1863.
Zhao, C. H.; Wakamiya, A.; Inukai, Y.; Yamaguchi, S. J. Am. Chem. Soc. 2006, 128, 15934.
Wakamiya, A.; Mori, K.; Yamaguchi, S. Angew. Chem., Int. Ed. 2007, 46, 4273.
(a) Weber, L. Coord. Chem. Rev. 2001, 215, 39. (b) Weber, L. Coord. Chem. Rev. 2008, 252, 1. (c) Weber, L.; Böhling, L. Coord. Chem. Rev. 2015, 284, 236. (d) Weber, L. Eur. J. Inorg. Chem. 2012, 5595. (e) Yamashita, M.; Nozaki, K. J. Synth. Org. Chem. Jpn. 2010, 68, 359. (f) Yamashita, M.; Nozaki, K. Bull. Chem. Soc. Jpn. 2008, 81, 1377. (h) Weber, L.; Wartig, H. B.; Stammler, H.-G.; Neumann, B. Organometallics 2001, 20, 5248. (i) Weber, L.; Wartig, H. B.; Stammler, H.-G.; Neumann, B. Z. Anorg. Allg. Chem. 2001, 627, 2663. (j) Weber, L.; Domke, I; Greschner, W.; Miqueu, K.; Chrostowska, A.; Baylère, P. Organometallics 2005, 24, 5455.
(a) Habereder, T.; Nöth, H. Appl. Organomet. Chem. 2003, 17, 525. (b) Weber, L.; Domke, I.; Kahlert, J.; Stammler, H.-G. Eur. J. Inorg. Chem. 2006, 3419. (c) Weber, L.; Rausch, A; Stammler, H.-G.; Neumann, B. Z. Anorg. Allg. Chem. 2004, 630, 2657. (d) Weber, L.; Förster, J.; Stammler, H.-G.; Neumann, B. Eur. J. Inorg. Chem. 2006, 5048. (e) Weber, L.; Schnieder, M.; Maciel, T. C.; Wartig, H.; Schimmel, M.; Boese, R.; Bläser, D. Organometallics 2000, 19, 5791. (f) Murphy, J. M.; Lawrence, J. D.; Kawamura, K.; Incarvito, C.; Hartwig, J. F. J. Am. Chem. Soc. 2006, 128, 13684. (g) Segawa, Y.; Yamashita, M.; Nozaki, K. Science 2006, 314, 113. (h) Marder, T. B. Science 2006, 314, 69. (i) Braunschweig, H. Angew.Chem., Int. Ed. 2007, 46, 1946. (j) Segawa, Y.; Yamashita, M.; Nozaki, K. Angew. Chem., Int. Ed. 2007, 46, 6710. (k) Kajiwara, T.; Terebayashi, T.; Yamashita, M.; Nozaki, K. Angew. Chem., Int. Ed. 2008, 47, 606. (l) Segawa, Y.; Suzuki, Y; Yamashita, M.; Nozaki, K. J. Am. Chem. Soc. 2008, 130, 16069. (m) Yamashita, M.; Suzuki, Y.; Segawa, Y.; Nozaki, K. Chem. Lett. 2008, 37, 802. (n) Terebayashi, T.; Kajiwara, T.; Yamashita, M.; Nozaki, K. J. Am. Chem. Soc. 2009, 131, 14162. (o) Nozaki, K.; Arami, Y.; Yamashita, M.; Ueng, S.-H.; Malacria, M.; Lacôte, E.; Curran, D. P. J. Am. Chem. Soc. 2010, 132, 11449. (p) Hayashi, Y.; Segawa, Y.; Yamashita, M.; Nozaki, K. Chem. Commun. 2011, 47, 5888. (q) Segawa, Y.; Yamashita, M.; Nozaki, K. Organometallics 2009, 28, 6234. (r) Hasegawa, M.; Segawa, Y.; Yamashita, M.; Nozaki, K. Angew. Chem., Int. Ed. 2012, 51, 6956. (s) Dettenrieder, H.; Dietrich , H. M.; Schädle, C.; Maichle-Mössmer, C.; Törnroos, K. W.; Anwander, R. Angew. Chem., Int. Ed. 2012, 51, 4461. (t) Protochenko, A. V.; Birjkumar, K. H.; Dange, D.; Schwarz, A. D.; Vidovic, D.; Jones, C.; Kaltsoyannis, N.; Mountford, P.; Aldridge, S. J. Am. Chem. Soc. 2012, 134, 6500. (u) Saleh, L. M. A.; Nirjkumar, K. H.; Protochenko, A. V.; Schwarz, A. D.; Aldridge, S.; Jones, C.; Kaltsoyannis, N.; Mountford, P. J. Am. Chem. Soc. 2011, 133, 3836. (v) Li, S.; Cheng, J.; Chen, Y.; Nishiura, M.; Hou, Z. Angew. Chem., Int. Ed. 2011, 50, 6360. (w) Tian, D.; Jiang, J.; Hu, H.; Zhang, J.; Cui, C. J. Am. Chem. Soc. 2012, 134, 14666. (x) Hinchcliffe, A.; Mair, F. S.; McInnes, E. J. L.; Pritchard, R. G.; Warren, J. E. Dalton Trans. 2008, 222. (y) Giziroglu, E.; Donnadieu, B.; Bertrand, G. Inorg. Chem. 2008, 47, 9751.
(a) Weber, L.; Penner, A.; Stammler, H.-G.; Neumann, B. Z. Anorg. Allg. Chem. 2007, 633, 563. (b) Weber, L.; Eickhoff, D.; Werner, V.; Böhling, L.; Schwedler, S.; Chrostowska, A.; Dargelos, A.; Maciejczyk, M.; Stammler, H.-G.; Neumann, B. Dalton Trans. 2011, 40, 4434. (c) Schwedler, S.; Eickhoff, D.; Brockhinke, R.; Cherian, D.; Weber, L.; Brockhinke, A. Phys. Chem. Chem. Phys. 2011, 13, 9301. (d) Weber, L.; Werner, V.; Domke, I.; Stammler, H.-G.; Neumann, B. Dalton Trans. 2006, 3777. (e) Chrostowska, A.; Maciejczyk, M.; Dargelos, A.; Baylère, P.; Weber, L.; Werner, V.; Eickhoff, D.; Stammler, H.-G.; Neumann, B. Organometallics 2010, 29, 5192.
Weber, L.; Werner, V.; Fox, M. A.; Marder, T. B.; Schwedler, S.; Brockhinke, A.; Stammler, H.-G.; Neumann, B. Dalton Trans. 2009, 2823.
(a) Weber, L.; Halama, J.; Böhling, L.; Brockhinke, A.; Chrostowska, A.; Darrigan, C.; Dargelos, A.; Stammler, H.-G.; Neumann, B. Eur. J. Inorg. Chem. 2013, 4268. (b) Weber, L.; Halama, J.; Hanke, K.; Böhling, L.; Brockhinke, A.; Stammler, H.-G.; Neumann, B.; Fox, M. A. Dalton Trans. 2014, 43, 3347.
(a) Marsden, J. A.; Miller, J. J.; Shirtcliff, L. D.; Haley, M. M. J. Am. Chem. Soc. 2005, 127, 2464. (b) Grabarz, A. M.; Laurent, A. D; Jędrzejewska, B.; Zakrewska, A.; Jacquemin, D.; Ośmiałowski, B. J. Org. Chem. 2016, 81, 2280.
(a) Weber, L.; Eickhoff, D.; Marder, T. B.; Fox, M. A.; Low, P. J.; Dwyer, A. D.; Tozer, D. J.; Schwedler, S.; Brockhinke, A.; Stammler, H.-G.; Neumann, B. Chem.–Eur. J. 2012, 18, 1369. (b) Weber, L.; Eickhoff, D.; Kahlert, J.; Böhling, L.; Brockhinke, A.; Stammler, H.-G.; Neumann, B.; Fox, M. A. Dalton Trans. 2012, 41, 10328.
(a) Bosdet, M. J. D.; Piers, W. E. Can. J. Chem. 2009, 87, 8. (b) Campbell, P. G.; Marwitz, A. J.; Liu, S.-Y. Angew. Chem., Int. Ed. 2012, 51, 6074. (c) Abbey, E. R.; Liu, S.-Y. Org. Biomol. Chem. 2013, 11, 2060. (d) Schäfer, M.; Schäfer, J.; Dewhurst, R. D.; Ewing, W. C.; Krahfuß, M.; Kuntze- Fechner, M. W.; Wehner, M.; Lambert, C.; Braunschweig, H. Chem.–Eur. J. 2016, 22, 8603
Pelter, A.; Smith, K.; Brown, H. C. Borane Reagents; Academic Press: London, 1988, p. 428.
Bergmann, J.; Venemalm, L. J. Org. Chem. 1992, 57, 2495.
An, Z.; Odom, S. A.; Kelley, R. F.; Huang, C.; Zhang, X.; Barlow, S.; Padilha, L. A.; Fu, J.; Webster, S.; Hagan, D. J.; Van Stryland, E. W.; Wasielewsky, M. R.; Marder, S. R. J. Phys. Chem. A 2009, 113, 5585.
Blackburn, B. K.; Lee, A.; Baier, M.; Kohl, B.; Olivere, A. G.; Matamaros, R.; Robarge, K. D.; Mc Dowell, R. S. J. Med. Chem. 1997, 40, 717.
Weber, L.; Dobbert, E.; Stammler, H.-G.; Neumann, B.; Boese, R.; Bläser, D. Chem. Ber. 1997, 130, 705.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64A, 112.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, revision B.01; Gaussian, Inc.: Wallingford, 2009.
Raghavachari, K.; Binkley, J. S.; Seeger, R.; Pople, J. A. J. Chem. Phys. 1980, 72, 650.
(a) Parr, R. G.; Yang, W. Functional Theory of Atoms and Molecules; Oxford University Press: New York, 1989. (b) Frisch, M. J.; Trucks, G. W.; Cheeseman, J. R. In Recent Development and Applications of Modern Density Functional Theory, Theoretical and Computational Chemistry; Semminario, J. M., Ed.; Elsevier: Amsterdam–Lausanne–New York–Oxford–Shannon–Tokyo, 1996, Vol. 4, p. 679. (c) Limacher, P. A.; Mikkelsen, K. V.; Lüthi, H. P. J. Chem. Phys. 2009, 130, 194114. (d) Kobayashi, R.; Amos, R. D. Chem. Phys. Lett. 2006, 420, 106. (e) Jacquemin, D.; Perpète, E. A.; Scalmani, G.; Frisch, M. J.; Kobayashi, R.; Adamo, C. J. Chem. Phys. 2007, 126, 144105.
(a) Becke, A. D. Phys. Rev. 1988, 38(6), 3098. (b) Becke, S. D. J. Chem. Phys. 1993, 98(7), 5648. (c) Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. 1988, B37(2), 785. (d) Yanai, T.; Tew, D.; Handy, N. Chem. Phys. Lett. 2004, 393(1–3), 51.
(a) Joantéguy, S.; Pfister-Guillouzo, G.; Chermette, H. J. Phys. Chem. 1999, 103(18), 3505. (b) Chrostowska, A.; Miqueu, K.; Pfister-Guillouzo, G.; Briard, E.; Levillain, J.; Ripoll, J.-L. J. Mol. Spectrosc. 2001, 205(2), 323. (c) Bartnik, R.; Baylère, P.; Chrostowska, A.; Galindo, A.; Lesniak, S.; Pfister-Guillouzo, G. Eur. J. Org. Chem. 2003, (13), 2475.
(a) Stratmann, R. E.; Scuseria, G. E.; Frisch, M. J. J. Chem. Phys. 1998, 109(19), 8218. (b) Casida, M. E.; Jamorski, C.; Casida, K. C.; Salahub, D. R. J. Chem. Phys. 1998, 108(11), 4439. (c) Lemierre, V.; Chrostowska, A.; Dargelos, A.; Chermette, H. J. Phys. Chem. A 2005, 109(37), 8348.
Varetto, U. Molekel 4.3; Swiss National Supercomputing Centre: Manno (Switzerland).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(1), 54–65
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2158 kb)
Rights and permissions
About this article
Cite this article
Weber, L., Eickhoff, D., Chrostowska, A. et al. Synthesis, structure, and properties of luminescent diazaborole and indole systems. Chem Heterocycl Comp 53, 54–65 (2017). https://doi.org/10.1007/s10593-017-2021-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-2021-0