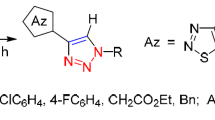
2-(1,2,3-Thiadiazol-5-yl)enamines and 3-(1,2,3-triazol-4-yl)enaminones react with arylhydroxamoyl chlorides at room temperature with the exclusive formation of 3-aryl-4-(1,2,3-thiadiazol-5-yl)- and [4-(1,2,3-triazol-4-yl)carbonyl]isoxazoles in high yields. The proposed mechanism includes in situ generation of nitrile oxides, which participate in the (3+2)-dipolar cycloaddition reactions leading to the formation of the isoxazole ring.


Similar content being viewed by others
Notes
Successively added methylamine hydrochloride (2.7 g, 40 mmol) and NaOH (1.2 g, 30 mmol) can be used as methylamine (2) source.
The assignment was done on the basis of the data of a 2D HMBC experiment.
References
Giomi, D.; Cordero, F. M.; Machetti, F. In Comprehensive Heterocyclic Chemistry III; Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V. Pergamon Press: Oxford, 2008, Vol. 4, p. 365.
Gehling, V. S.; Hewitt, M. C.; Vaswani, R. G.; Leblanc, Y.; Côté, A.; Nasveschuk, C. G.; Taylor, A. M.; Harmange, J.-C.; Audia, J. E.; Pardo, E.; Joshi, S.; Sandy, P.; Mertz, J. A.; Sims, R. J.; Bergeron, L.; Bryant, B. M.; Bellon, S.; Poy, F.; Jayaram, H.; Sankaranarayanan, R.; Yellapantula, S.; Srinivasamurthy, N. B.; Birudukota, S.; Albrecht, B. K. ACS Med. Chem. Lett. 2013, 4, 835.
Di Nunno, L.; Vitale, P.; Scilimati, A.; Tacconelli, S.; Patrignani, P. J. Med. Chem. 2004, 47, 4881.
Galenko, A. V.; Khlebnikov, A. F.; Novikov, M. S.; Pakalnis, V. V.; Rostovskii, N. V. Russ. Chem. Rev. 2015, 84, 335.
Girardin, M.; Dolman, S. J.; Lauzon, S.; Ouellet, S. G.; Hughes, G.; Fernandez, P.; Zhou, G.; O'Shea, P. D. Org. Process Res. Dev. 2011, 15, 1073.
Brodney, M. A.; Beck, E. M.; Butler, C. R.; Barreiro, G.; Johnson, E. F.; Riddell, D.; Parris, K.; Nolan, C. E.; Fan, Y.; Atchison, K.; Gonzales, C.; Robshaw, A. E.; Doran, S. D.; Bundesmann, M. W.; Buzon, L.; Dutra, J.; Henegar, K.; La Chapelle, E.; Hou, X.; Rogers, B. N.; Pandit, J.; Lira, R.; Martinez-Alsina, L.; Mikochik, P.; Murray, J. C.; Ogilvie, K.; Price, L.; Sakya, S. M.; Yu, A.; Zhang, Y.; O'Neill, T. J. Med. Chem. 2015, 58, 3223.
Amegadzie, A. K.; Gardinier, K. M.; Hembre, E. J. Hong, J. E.; Jungheim, L. N.; Muehl, B. S.; Remick, D. M.; Robertson, M. A.; Savin, K. A. WO Patent 2003091226.
Leban, J.; Tasler, S.; Saeb, W.; Chevrier, C. WO Patent 2012101263; Chem. Abstr. 2012, 158, 17532d.
Bakulev, V. A.; Tarasov, E. V.; Morzherin, Y. Y.; Luyten, I.; Toppet, S.; Dehaen, W. Tetrahedron 1998, 54, 850
Lebedev, A. T.; Dankova, E. F.; Mokrushin, V. S.; Petrosyan, V. S. Tetrahedron 1989, 45, 7329.
Bakulev, V. A.; Mokrushin, V. S. Chem. Heterocycl. Compd. 1986, 22, 811. [Khim. Geterotsikl. Soedin. 1986, 1011.]
Buettelmann, B.; Han, B.; Knust, H.; Thomas, A. WO Patent 2007074078; Chem. Abstr. 2007, 147, 143428.
Thorarensen, A.; Ruble, C. J.; Fisher, J. F.; Romero, D. L.; Beauchamp, T. J.; Northuis, J. M. WO Patent 2004018428; Chem. Abstr., 2004, 140, 235498.
Eiden, F.; Patzelt, G. Arch. Pharm. 1986, 319, 242.
Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V. V.; Noodleman, L.; Sharpless, K. B.; Fokin, V. V. J. Am. Chem. Soc. 2005, 127, 210.
Bakulev, V. A.; Efimov, I. V.; Belyaev, N. A.; Rozin, Yu. A.; Volkova, N. N.; El'tsov, O. S. Chem. Heterocycl. Compd. 2012, 47, 1593. [Khim. Geterotsikl. Soedin. 2011, 1900.]
Al-Shiekh, M. A.; Salah El-Din, A. M.; Hafez, E. A.; Elnagdi, M. H. J. Chem. Res., Synop. 2004, 174.
Jones, R. C. F.; Dawson, C. E.; O'Mahony, M. J. Synlett 1999, 873.
Jones, R. C. F.; Pillainayagam, T. A. Synlett 2004, 2815.
Bakulev, V. A.; Efimov, I. V.; Belyaev, N. A.; Zhidovinov, S. S.; Rozin, Yu. A.; Volkova, N. N.; Khabarova, A. A.; El'tsov, O. S. Chem. Heterocycl. Compd. 2013, 48, 1880. [Khim. Geterotsikl. Soedin. 2012, 2002.]
Harjinder, S; Jayant, S; Jitender, K. RSC Adv. 2013, 3, 22360.
Krompiec, S.; Bujak, P.; Szczepankiewicz, W. Tetrahedron Lett. 2008, 49, 6071.
Bujak, P.; Krompiec, S.; Malarz, J.; Krompiec, M.; Filapek, M.; Danikiewicz, W.; Kania, M.; Gebarowska, K.; Grudzka, I. Tetrahedron 2010, 66, 5972.
Rao, K. R.; Nageswar, Y. V. D.; Sattur, P. B. J. Heterocycl. Chem. 1989, 26, 255.
Peša, N.; Welch, C. J.; Boa, A. N. J. Heterocycl. Chem. 2005, 42, 599.
Caramella, P. A.; Corsico, DC.; Corsaro, A.; Monto, D. D.; Albini, F. M. Tetrahedron 1982, 38, 173.
Regitz, M.; Liedhegener, A. Chem. Ber. 1966, 99, 3128.
Lemercier, B. C.; Pierce, J. G. J. Org. Chem. 2014, 79, 2321.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Melo, J. O. F.; Ratton, P. M.; Augusti, R.; Donnici, C. L. Synth. Commun. 2004, 34, 369.
Augusti, R.; Kascheres, C. J. Org. Chem. 1993, 58, 7079.
This work was supported financially by the Russian Foundation for Basic Research (grant 14-03-01033) and the Ministry of Education and Science of the Russian Federation (State assignment 4.1626.2014/K).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(9), 743–749
Rights and permissions
About this article
Cite this article
Efimov, I.V., Shafran, Y.M., Volkova, N.N. et al. Synthesis of Assemblies of Isoxazole and Azoles Based on 1,3-Dipolar Cycloaddition Reaction of Enamines with Nitrile Oxides. Chem Heterocycl Comp 52, 743–749 (2016). https://doi.org/10.1007/s10593-016-1958-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1958-8