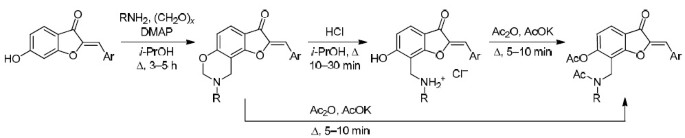
Aminomethylation of 6-hydroxyaurones with primary amines was used to synthesize 2-benzylidene-8,9-dihydro-7H-furo[2,3-f][1,3]-benzoxazin-3(2H)-one derivatives, while opening of 1,3-oxazine ring in the presence of acid gave secondary amines containing a 6-hydroxyaurone moiety. Acetylation of 2-benzylidene-8,9-dihydro-7H-furo[2,3-f][1,3]benzoxazin-3(2H)-ones was also accompanied by opening of 1,3-oxazine ring.


Similar content being viewed by others
References
Tramontini, M. Synthesis 1973, 703.
(a) Shridhar, D. R.; Sastry, C. V. R.; Lal, B.; Raddi, G. S.; Bhopale, K. K.; Khokar, R. S.; Tripathi, K. Indian J. Chem.,Sect. B: Org. Chem. Incl. Med. Chem. 1980, 19, 1065. (b) Gadre, J. N.; Raote, P. S. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1993, 32, 1285. (c) Desai, R. B. J. Org. Chem. 1961, 26, 5251. (d) Patel, M. G.; Sethna, S. J. Indian Chem. Soc. 1962, 39, 595. (e) Kontogiorgis, C. A.; Hadjipavlou-Litina, D. J. J. Med. Chem. 2005, 48, 6400.
Nagorichna, I. V.; Garazd, M. M.; Garazd, Ya. L.; Khilya, V. P. Chem. Nat. Compd. 2007, 43, 15. [Khim. Prirod. Soedin. 2007, 14.]
(a) Bondarenko, S. P.; Frasinyuk, M. S.; Khilya, V. P. Chem. Nat. Compd. 2009, 45, 492. [Khim. Prirod. Soedin. 2009, 418.] (b) Bondarenko, S. P.; Frasinyuk, M. S.; Khilya, V. P. Chem. Heterocycl. Compd. 2010, 46, 146. [Khim. Geterotsikl. Soedin. 2010, 180.] (c) Bondarenko, S. P.; Frasinyuk, M. S.; Galaev, A. I.; Vinogradova, V. I. Chem. Nat. Compd. 2012, 48, 234. [Khim. Prirod. Soedin. 2012, 212.] (d) Gorbulenko, N. V.; Tkachuk, T. M.; Shokol, T. V.; Semeniuchenko, V. V.; Turov, A. V.; Khilya, V. P. Chem. Heterocycl. Compd. 2007, 43, 569. [Khim. Geterotsikl. Soedin. 2007, 683.] (e) Frasinyuk, M. S.; Bondarenko, S. P.; Khilya, V. P.; Liu, C.; Watt, D. S.; Sviripa, V. M. Org. Biomol. Chem. 2015, 13, 1053.
Jogi, P. S.; Meshram, J. S.; Sheikh, J. Lett. Drug Design Discov. 2013, 10, 283.
Kontogiorgis, C.; Nicolotti, O.; Mangiatordi, G. F.; Tognolini, M.; Karalaki, F.; Giorgio, C.; Patsilinakos, A.; Carotti, A.; Hadjipavlou-Litina, D.; Barocelli, E. J. Enzyme Inhib. Med. Chem. 2015, 30, 925.
Kuehne, M. E.; Konopka, E. A. J. Med. Pharm. Chem. 1962, 5, 257.
(a) Wang, D.; Hou, L.; Wu, L.; Yu, X. Chem. Pharm. Bull. 2012, 60, 513. (b) Chen, Y.; Cass, S. L.; Kutty, S. K.; Yee, E. M. H.; Chan, D. S. H.; Gardner, C. R.; Vittorio, O.; Pasquier, E.; Black, D. S.; Kumar, N. Bioorg. Med. Chem. Lett. 2015, 25, 5377.
Rodriguez Arza, C.; Froimowicz, P.; Ishida, H. RSC Adv. 2015, 5, 97855.
Haudecoeur, R.; Boumendjel, A. Curr. Med. Chem. 2012, 19, 2861.
Kolehmainen, E. Annu. Rep. NMR Spectrosc. 2003, 49, 1.
(a) Carrasco, M. P.; Newton, A. S.; Gonçalves, L.; Góis, A.; Machado, M.; Gut, J.; Nogueira, F.; Hänscheid, T.; Guedes, R. C.; dos Santos, D. J. V. A.; Rosenthal, P. J.; Moreira, R. Eur. J. Med. Chem. 2014, 80, 523. (b) Nakano, H.; Saito, N.; Parker, L.; Tada, Y.; Abe, M.; Tsuganezawa, K.; Yokoyama, S.; Tanaka, A.; Kojima, H.; Okabe, T.; Nagano, T. J. Med. Chem. 2012, 55, 5151.
Frasinyuk, M. S.; Mrug, G. P.; Bondarenko, S. P.; Sviripa, V. M.; Zhang, W.; Cai, X.; Fiandalo, M.; Mohler, J. L.; Liu, C.; Watt, D. S. Org. Biomol. Chem. 2015, 13, 11292.
(a) Shin, S. Y.; Shin, M. C.; Shin, J.-S.; Lee, K.-T.; Lee, Y. S. Bioorg. Med. Chem. Lett. 2011, 21, 4520. (b) Zhao, W.; Sun, J.; Xiang, H.; Zeng, Y.-Y.; Li, X.-B.; Xiao, H.; Chen, D.-Y.; Ma, R. L. Bioorg. Med. Chem. 2011, 19, 3192. (c) Lee, Y. H.; Shin, M. C.; Yun, Y. D.; Shin, S. Y.; Kim, J. M.; Seo, J. M.; Kim, N.-J.; Ryu, J. H.; Lee, Y. S. Bioorg. Med. Chem. 2015, 23, 231.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(8), 592–600
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 12655 kb)
Rights and permissions
About this article
Cite this article
Popova, A.V., Bondarenko, S.P. & Frasinyuk, M.S. Synthesis and properties of 2-benzylidene-8,9-dihydro-7H-furo[2,3-f][1,3]benzoxazin-3(2H)-one derivatives. Chem Heterocycl Comp 52, 592–600 (2016). https://doi.org/10.1007/s10593-016-1937-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1937-0



