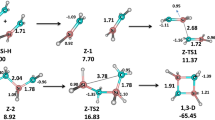
Three Si-substituted silatranes were synthesized in order to rectify their structure with particular attention to the length of the N–Si intramolecular dative bond. DFT calculations of potential energy profile as a function of the N–Si distance and the structure of HOMO were considered. The latter was found to vary its localization depending on the electron-donating properties of the substituent and the N–Si distance.






Similar content being viewed by others
Notes
Unpublished results.
References
Baltkais, N. J.; Voronkov, M. G.; Zelchan, G. I. Izv. Akad. Nauk Latv. SSR, Ser. Khim. 1964, 102; Chem. Abstr. 1964, 61, 57120.
Voronkov, M. G.; Dyakov, V. M.; Kirpichenko, S. V. J. Organomet. Chem. 1982, 233, 1.
Pestunovich, V.; Kirpichenko, S.; Voronkov, M. In The Chemistry of Organic Silicon Compounds; Rappoport, Z.; Apeloig, Y., Eds; John Wiley & Sons: Chichester, 1998, Vol. 2, p. 1447.
Verkade, J. G. Coord. Chem. Rev. 1994, 137, 233.
Tandura, S. N.; Voronkov, M. G.; Alekseev, N. V. Top. Curr. Chem. 1986, 131, 99.
Trofimov, A. B.; Zakrzewski, V. G.; Dolgounitcheva, O.; Ortiz, J. V.; Sidorkin, V. F.; Belogolova, E. F. ; Belogolov, M.; Pestunovich, V. A. J. Am. Chem. Soc. 2005, 127, 986.
Sidorkin, V. F.; Pestunovich, V. A.; Voronkov, M. G. Dokl. Akad. Nauk SSSR 1977, 235, 1363.; Chem. Abstr. 1977, 87, 173187.
Sidorkin, V. F.; Pestunovich, V. A.; Voronkov, M. G. Russ. Chem. Rev. 1980, 49, 414. [Usp. Khim. 1980, 49, 789.]
Broka, K.; Stradiņš, J.; Glezer, V.; Zelčāns, G.; Lukevics, E. J. Electroanal. Chem. 1993, 351, 199.
Broka, K.; Glezer, V.; Stradins, J.; Zelcans, G. Zh. Obshch. Khim. 1991, 61, 1374.
Jouikov, V. ECS Trans. 2010, 28(12), 5.
Peureux, C.; Jouikov, V. Chem.–Eur. J. 2014, 20, 9290.
Soualmi, S.; Ignatovich, L.; Jouikov, V. Appl. Organomet. Chem. 2010, 24, 865.
Ignatovich, L.; Jouikov, V. J. Organomet. Chem. 2014, 751, 546.
Zöller, T.; Dietz, C.; Iovkova-Berends, L.; Karsten, O.; Bradtmöller, G.; Wiegand, A.-K.; Wang, Y.; Jouikov, V.; Jurkschat, K. Inorg. Chem. 2012, 51, 1041.
Puri, J. K.; Singh, R.; Chahal, V. K. Chem. Soc. Rev. 2011, 40, 1791.
Voronkov, M. G.; Dyakov, V. M.; Kirpichenko, S. V. J. Organomet. Chem. 1982, 233, 1.
Voronkov, M. G.; Dyakov, V. M. Silatranes [in Russian]; Nauka: Novosibirsk, 1978.
Voronkov, M. G.; Kuznetsova, G. A.; Baryshok, V. P. Rus. J. Gen. Chem. 1983, 53, 1512. [Zh. Obshch. Khim. 1983, 53, 1682.]
Frye, C. L.; Vogel, G. E.; Hall, J. A. J. Am. Chem. Soc. 1961, 83, 996.
Cerveau, G.; Chuit, C.; Corriu, R. J. P.; Reye, C. J. Organomet. Chem. 1987, 328, C17.
Zhuo, R.; Lu, Z.-R.; Liao, J.; Shen, L.-F. J. Organomet. Chem. 1993, 446, 107.
Lukevics, E.; Ignatovich, L. M. Chem. Heterocycl. Compd. 1992, 28, 603. [Khim. Geterotsikl. Soedin. 1992, 725.]
Corriu, R. J. In Frontiers of Organosilicon Chemistry; Bassindale, A. R.; Gaspar P. P., Eds.; RSC: Cambridge, 1991, p.126 .
Armarego, W. L. E.; Chai, C. L. L. Purification of Organic Chemicals; Butterworth-Heinemann: Amsterdam, 2003, 5th d.; p. 215.
Brook, M. A. Silicon in Organic, Organometallic, and Polymer Chemistry; John Wiley & Sons: Toronto, 2000, p. 31.
Chernyshev, E. P.; Knazev, S. P.; Kirin, V. N.; Vasiliev, I. M.; Alekseev, N. V. Rus. J. Gen. Chem. 2004, 74, 58. [Zh. Obshch. Khim. 2004, 74, 65.]
Tomasi, J.; Mennucci, B.; Cammi, R. Chem. Rev. 2005, 105, 2999.
Amatore, C. In Organic Electrochemistry; Lund, H.; Hammerich, O., Eds.; Marcel Dekker: New York, 2001, 4th ed., p. 1.
Belogolova, E. F.; Vakul'skaya, T. I.; Sidorkin, V. F. Phys. Chem. Chem. Phys. 2015, 17, 12735.
Sidorkin, V. F.; Belogolova, E. F.; Doronina, E. P. Phys. Chem. Chem. Phys. 2015, 17, 26225.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, J. A.; Vreven, T., Jr.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O. ; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian 03, Revision B.01; Gaussian, Inc.: Pittsburgh, 2003.
Brennan, B. J., Gust, D., Brudvig, G. W. Tetrahedron Lett. 2014, 55, 1062.
Voronkov, M. G.; Zelcan, G. I. Chem. Heterocycl. Compd. 1969, 5, 34. [Khim. Geterotsikl. Soedin. 1969, 43.]
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, A71, 3.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
van der Sluis, P.; Spek, A. L. Acta Crystallogr., Sect. A: Found. Crystallogr. 1990, A46, 194.
Spek, A. L. J. Appl. Crystallogr. 2003, 36, 7.
The authors gratefully acknowledge the support of this research by InnovaBalt program and the Latvian Council for Science (Project 225/2012).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Lubova Ignatovica is Deceased
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(8), 546–550
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 241 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Romanovs, V., Spura, J. et al. Anisyl-, aminophenyl-, and naphthylmethylsilatranes revisited. Chem Heterocycl Comp 52, 546–550 (2016). https://doi.org/10.1007/s10593-016-1928-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1928-1