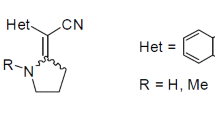
A new, highly efficient method was developed for the synthesis of (3-indolyl)acetonitriles by reduction of readily available (3-indolyl)hydroxamic acids with phosphorus trichloride. The nitriles obtained according to this method are of significant interest for structure-activity studies of potential anticancer agents.
Similar content being viewed by others
References
(a) Yang, L.; Wang, G.; Wang, M.; Jiang, H.; Chen, L.; Zhao, F.; Qiu, F. Fitoterapia 2014, 95, 175. (b) Zhang, J.; Wang, J.-D.; Liu, C.-X.; Yuan, J.-H.; Wang, X.-J.; Xiang, W.-S. Nat. Prod. Res. 2014, 28, 431. (c) Pedras, M. S. C.; Yaya, E. E. Chem. Biodiversity 2014, 11, 910. (d) Chen, M.; Gan, L.; Lin, S.; Wang, X.; Li, L.; Li, Y.; Zhu, C.; Wang, Y.; Jiang, B.; Jiang, J.; Yang, Y.; Shi, J. J. Nat. Prod. 2012, 75, 1167. (e) Wu, Q.; Bang, M.-H.; Lee, D.-Y.; Cho, J.-G.; Jeong, R.-H.; Shrestha, S.; Lee, K.-T.; Chung, H.-G.; Ahn, E.-M.; Baek, N.-I. Chem. Nat. Comp. 2012, 48, 281. [Khim. Prirod. Soedin. 2012, 251.]
(a) Cho, E.-J.; Shin, J.-S.; Chung, K.-S.; Lee, Y. S.; Cho, Y.-W.; Baek, N.-I.; Chung, H.-G.; Lee, K.-T. J. Agric. Food Chem. 2012, 60,7398. (b) Wu, Y.; Zhang, Z.-X.; Hu, H.; Li, D.; Qiu, G.; Hu, X.; He, X. Fitoterapia 2011, 82, 288.
Aksenov, A. V.; Smirnov, A. N.; Magedov, I. V.; Reisenauer, M. R.; Aksenov, N. A.; Aksenova, I. V.; Pendleton, A. L.; Nguyen, G.; Johnston, R. K.; Rubin, M.; De Carvalho, A.; Kiss, R.; Mathieu, V.; Lefranc, F.; Correa, J.; Cavazos, D. A.; Brenner,. J.; Bryan, B. A.; Rogelj, S.; Kornienko, A.; Frolova, L. V. J. Med. Chem. 2015, 58, 2206.
(a) Aksenov, A. V.; Smirnov, A. N.; Aksenov, N. A.; Aksenova, I. V.; Frolova, L. V.; Kornienko, A.; Magedov, I. V.; Rubin, M. Chem. Commun. 2013, 49, 9305. (b) Aksenov, A. V.; Smirnov, A. N.; Aksenov, N. A.; Aksenova, I. V.; Bijieva, A. S.; Rubin, M. Org. Biomol. Chem. 2014, 12, 9786. (c) Aksenov, A. V.; Smirnov, A. N.; Aksenov, N. A.; Aksenova, I. V.; Matheny, J. P.; Rubin, M. RSC Adv. 2015, 5, 8647.
Aksenov, A. V.; Aksenov, N. A.; Dzhandigova, Z. V.; Aksenov, D. A.; Rubin, M. RSC Adv. 2015, 5, 106492.
(a) Liguori, A.; Sindona, G.; Romeo, G.; Uccella, N. Synthesis 1987, 168. (b) Ashworth, I. W.; Bowden, M. C.; Dembofsky, B.; Levin, D.; Moss, W.; Robinson, E.; Szczur, N.; Virica, J. Org. Proc. Res. Dev. 2003, 7, 74.
(a) Middleton, W. J. J. Org. Chem. 1983, 48, 3845. (b Thompson, H. W.; Rashid, S. Y. J. Org. Chem. 2002, 67, 2813. (c) Reiner, J. E.; Siev, D. V.; Araldi, G.-L.; Cui, J. J.; Ho, J. Z.; Reddy, K. M.; Mamedova, L.; Vu, P. H.; Lee, K.-S. S.; Minami, N. K.; Gibson, T. S.; Anderson, S. M.; Bradbury, A. E.; Nolan, T. G.; Semple, J. E. Bioorg. Med. Chem. Lett. 2002, 12, 1203.
This study was performed with financial support from the Russian Science Foundation (grant No. 14-13-01108).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(5), 299–302
Rights and permissions
About this article
Cite this article
Aksenov, A.V., Aksenov, N.A., Dzhandigova, Z.V. et al. An efficient synthesis of (3-indolyl)acetonitriles by reduction of hydroxamic acids. Chem Heterocycl Comp 52, 299–302 (2016). https://doi.org/10.1007/s10593-016-1881-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1881-z