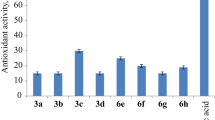
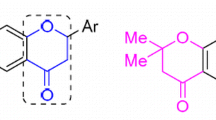
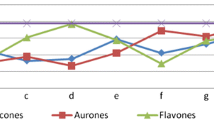
A new series of 3-hydroxy-2-[1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl]-4H-chromen-4-ones has been synthesized from substituted 2-hydroxyacetophenones and 1-phenyl-3-(thiophen-2-yl)-1H-pyrazole-4-carbaldehyde using NaOH and H2O2 by modified Algar–Flynn–Oyamada reaction under conventional and microwave irradiation conditions. In this method flavonols are synthesized without isolating chalcones in good yields (80–85%). The structures of the compounds were established on the basis of IR, 1H, 13C NMR and mass spectral and elemental analysis data. The synthesized compounds were screened for their antibacterial and antifungal activities.

Similar content being viewed by others
References
Primo, F. T.; Fröhlich, P. E. Acta Farm. Bonaerense 2005, 24, 421.
Riedel, R. Arzneim. Forsch. 1981, 31, 655.
Kumar, V.; Kaur, K.; Gupta, G. K.; Sharma, A. K. Eur. J. Med. Chem. 2013, 69, 735.
Nargund, L. V. G.; Hariprasad, V.; Reddy, G. R. N. Indian J. Pharm. Sci. 1993, 55, 1.
Sangani, C. B.; Makawana, J. A.; Zhang, X.; Teraiya, S. B.; Lin, L.; Zhu, H.-L. Eur. J. Med. Chem. 2014, 76, 549.
Sankappa Rai, U.; Isloor, A. M.; Shetty, P.; Pai, K. S. R.; Fun, H. K. Arabian J. Chem. 2015, 8, 317.
Blair, B.; Fatheree, R. P.; Fleury, M.; Gendron, R.; Hudson, R.; McKinnell, R. M.; Wilson, M. WO Patent 2011005674.
Secci, D.; Bolasco, A.; Chimenti, P.; Carradori, S. Curr. Med. Chem. 2011, 18, 5114.
Xu, L.; Zhang, X.; Li, X.; Wang, M.; Yuan, B. CN Patent 103232432.
Dong, F.; Chen, X.; Liu, X.; Xu, J.; Li, Y.; Shan, W.; Zheng, Y. J. Chromatogr. A 2012, 1262, 98.
Amr, A.-G.; Abdel-Latif, N. A.; Abdalla, M. M. Acta Pharm. 2006, 56, 203.
Oh, H. C.; Cho, J. H.; El-Gamal, M. KR Patent 2013010514.
Lee, H. I.; Le Hir de Fallois, L. P.; Timmons, P. R.; Cawthorne, W. G.; De Leon, A. P. WO Patent 2008005489.
Sherman, T. D.; Duke, M. V.; Clark, R. D.; Sanders, E. F.; Matsumoto, H.; Duke, O. S. Pestic. Biochem. Physiol. 1991, 40, 236.
Ragab, F. A.; Abdel Gawad, N. M.; Georgey, H. H.; Said, M. F. Eur. J. Med. Chem. 2013, 63, 645.
Mohy El-Din, M. M; Senbel, A. M.; Bistawroos, A. A.; El-Mallah, A.; Nour El-Din, N. A.; Bekhit, A. A.; Abd El Razik, H. A. Basic Clin. Pharmacol. Toxicol. 2011, 108, 263.
Hantoon, M. A. Minn. Med. 2001, 84, 102.
Zhang, X.; Li, X.; Allan, G. F.; Sbriscia, T.; Linton, O.; Lundeen, S. G.; Sui, Z. J. Med. Chem. 2007, 50, 3857.
Yang, X.; Jin, Y.; Liu, H.; Jiang, Y.; Fu, H. RSC Adv. 2012, 2, 11061.
Fong, T. M.; Heymsfield, S. B. Int. J. Obes. 2009, 33, 947.
Rademacher, P. M.; Woods, C. M.; Huang, Q.; Szklarz, G. D.; Nelson, S. D. Chem. Res. Toxicol. 2012, 25, 895.
Bhuiyan, M. H.; Khandkar, M. M. Pak. J. Sci. Ind. Res. 2009, 52, 180.
Murray, M. T. Encyclopedia of Nutritional Supplements; Random House: New York, 1996, p. 320.
Cimanga, K.; Ying, L.; De Bruyne, T.; Apers, S.; Cos, P.; Hermans, N.; Bakana, P.; Tona, L.; Kambu, K.; Kalenda, D. T.; Pieters, L.; Vanden Berghe, D.; Vlietinck, A. J. J. Pharm. Pharmacol. 2001, 53, 757.
Bandgar, B. P.; Patil, S. A.; Korbad, B. L.; Biradar, S. C.; Nile, S. N.; Khobragade, C. N. Eur. J. Med. Chem. 2010, 45, 3223.
Ercelen, S.; Klymchenko, A. S.; Demchenko, A. P. Anal. Chim. Acta 2002, 464, 273.
Klymchenko, A. S.; Avilov, S. V.; Demchenko, A. P. Anal. Biochem. 2004, 329, 43.
Franke, A. A.; Cooney, R. V.; Custer, L. J.; Mordan, L. J.; Tanaka, Y. Adv. Exp. Med. Biol. 1998, 439, 237.
Zwergel, C.; Valente, S.; Salvato, A.; Xu, Z.; Talhi, O.; Mai, A.; Silva, A.; Altucci, L.; Kirsch, G. Med. Chem. Commun. 2013, 4, 1571.
Chohan, Z. H.; Rauf, A.; Naseer, M. M.; Somra, M. A.; Supuran, C. T. J. Enzyme Inhib. Med. Chem. 2006, 21, 173.
Kumar, S.; Pandey, A. K. Sci. World J. 2013, 29, 162750.
Jain, A. C.; Gupta, S. M.; Sharma, A. Bull. Chem. Soc. Jpn. 1983, 56, 1267.
Girish, D. H.; Ashish, P. K.; Atish, H. R.; Rajesh, H. T.; Satish, S. B.; Mahendra, J. P.; Vandana, M. K. Med. Chem. Res. 2014, 23, 461.
Ashok, D.; Vijaya Lakshmi, B.; Ravi, S.; Ganesh, A. Med. Chem. Res. 2015, 24, 1487.
Serdiuk, I. E.; Roshal, A. D.; Błażejowski, J. Chem. Heterocycl. Compd. 2014, 50, 396. [Khim. Geterotsikl. Soedin. 2014, 431.]
Ashok, D.; Ravi, S.; Vijaya Lakshmi, B.; Ganesh, A. J. Serb. Chem. Soc. 2015, 80, 305.
Kiyani, H.; Albooyeh, F.; Fallahnezhad, S. J. Mol. Struct. 2015, 1091, 163.
Saha, K.; Mukherjee Pulok, K.; Mandal, S. C.; Pal, M.; Saha, B. P. Indian Drugs 1995, 32, 402.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information file to this article containing IR, 1H, 13C NMR and mass spectra of the synthesized compounds is available online at http://link.springer.com/journal/10593.
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(3), 172–176
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2395 kb)
Rights and permissions
About this article
Cite this article
Ashok, D., Kifah, M.A., Lakshmi, B.V. et al. Microwave-assisted one-pot synthesis of some new flavonols by modified Algar–Flynn–Oyamada reaction and their antimicrobial activity. Chem Heterocycl Comp 52, 172–176 (2016). https://doi.org/10.1007/s10593-016-1852-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1852-4