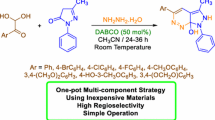
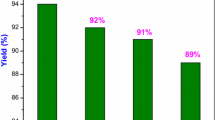
A new and convenient synthesis of 2-aryl-2,3-dihydroquinolin-4(1H)-ones has been described using the intramolecular cyclization of 2-aminochalcones catalyzed by 1-octyl-4-aza-1-azoniabicyclo[2.2.2]octane bromide ([C8dabco]Br). Recyclability of the catalyst, high yields, simple isolation of the products, and high atom economy are the noteworthy aspects of the protocol.


Similar content being viewed by others
References
(a) Shimokororiyama, M. In The Chemistry of Flavonoid Compounds; Geissaman, T. A., Ed.; Pergamon Press: NewYork, 1962, p. 286. (b) Harborne, J. B.; Williams, C. A. Nat. Prod. Rep. 1995, 12, 639.
Kalinin, V. N.; Shostakovskii, M. V.; Ponomarev, A. B. Tetrahedron Lett. 1992, 33, 373.
(a) The Flavonoids. Advances in Research Since 1980; Harborne, J. B., Ed.; Chapman and Hall: New York, 1988. (b) Flavonoids: Chemistry, Biochemistry and Applications; Andersen, Ø. M.; Markham, K. R., Eds.; Taylor & Francis Ltd.: London, 2006. (c) Chang, L. C.; Kinghorn, A. D. In Bioactive Compounds from Natural Sources: Isolation, Characterization and Biological Properties; Tringali, C., Ed.; Taylor & Francis Ltd.: London, 2001, p. 159.
(a) Xia, Y.; Yang, Z.-Y.; Xia, P.; Bastow, K. F.; Tachibana, Y.; Kuo, S.-C.; Hamel, E.; Hackl, T.; Lee, K.-H. J. Med .Chem. 1998, 41, 1155. (b) Laliberte, R.; Campbell, D. J.; Bruderlein, F. Can. J. Pharm. Sci. 1967, 2, 37.
Li, L.; Wang, H. K.; Kuo, S. C.; Wu, T. S.; Lednicer, D.; Lin, C.; Hamel, E.; Lee, K. H. J. Med. Chem. 1994, 37, 3400.
Xia, Y.; Yang, Z.-Y.; Xia, P.; Bastow, K. F.; Nakanishi, Y.; Lee, K.-H. Bioorg. Med. Chem. Lett. 2000, 10, 699.
Gao, F.; Johnson, K. F.; Schlenoff, J. B. J. Chem. Soc., Perkin Trans. 2 1996, 269.
(a) Xia, Y.; Yang, Z. Y.; Xia, P.; Bastow, K. F.; Tachibana, Y.; Kuo, S. C.; Hamel, E.; Hackl, T.; Lee, K. H. J. Med. Chem. 1998, 41, 1155. (b) Huang, L. J.; Hsieh, M. C.; Teng, C. M.; Lee, K. H.; Kuo, S. C. Bioorg. Med. Chem. 1998, 6, 1657. (c) Ko, T. C.; Hour, M. J.; Lien, J. C.; Teng, C. M.; Lee, K. H.; Kuo, S. C.; Huang, L. J. Bioorg. Med. Chem. 2001, 11, 279. (d) Xia, Y.; Yang, Z. Y.; Xia, P.; Hackl, T.; Hamel, E.; Mauger, A.; Wu, J. H.; Lee, K. H. J. Med. Chem. 2001, 44, 3932. (e) Xia, Y.; Yang, Z. Y.; Xia, P.; Hackl, T.; Hamel, E.; Mauger, A.; Wu, J. H.; Lee, K. H. Bioorg. Med. Chem. Lett. 2003, 13, 2891. (f) Hadjeri, M.; Peiller, E. L.; Beney, C.; Deka, N.; Lawson, M. A.; Dumontet, C.; Boumendjel, A. J. Med. Chem. 2004, 47, 4964. (g) Lai, Y. Y.; Huang, L. J.; Lee, K. H.; Xiao, Z.; Bastow, K. F.; Yamori, T.; Kuo, S. C. Bioorg. Med. Chem. 2005, 13, 265.
(a) Donnelly, J. A.; Farrell, D. F. Tetrahedron 1990, 46, 885. (b) Tokes, A. L.; Litkei, G. Synth. Commun. 1993, 23, 895. (d) Tokes, A. L.; Janzso, G. Synth. Commun. 1989, 19, 3159.
Donnelly, J. A.; Farrell, D. F. J. Org. Chem. 1990, 55, 1757.
Patonay, T.; Litkei, G.; Zsuga, M.; Kiss, A. Org. Prep. Proced. 1984, 16, 315.
Saravanamurugan, S.; Palanichamy, M.; Arabindoo, B.; Murugesan, V. J. Mol. Catal. A: Chem. 2004, 218, 101.
(a) Kloestra, K. R.; Bekkum, H. V. J. Chem. Soc. Chem. Commun. 1995, 1005. (b) Muthukrishnan, M.; Mujahid, M.; Punitharasu, V.; Dnyaneshwar, D. A. Synth. Commun. 2010, 40, 1391.
Kumar, D.; Patel, G.; Mishra, B. G.; Varma, R. S. Tetrahedron Lett. 2007, 49, 6974.
Kumar, D.; Patel, G.; Kumar, A.; Roy, R. K. J. Heterocycl. Chem. 2009, 46, 791.
Dittmer, C.; Raabe, G.; Hintermann, L. Eur. J. Org. Chem. 2007, 5886.
(a) Varma, R. S.; Saini, R. K. Synlett 1997, 857. (b) Kumar, K. H.; Perumal, P. T. Can. J. Chem. 2006, 84, 1079. (c) Tokes, A. L.; Litkei, G. Synth. Commun. 1993, 23, 895.
Kumar, K. H.; Muralidharan, D.; Perumal, P. T. Synthesis 2004, 63.
Ahmed, N.; Van Lier, J. E. Tetrahedron Lett. 2007, 48, 13.
Ahmed, N.; Van Lier, J. E. Tetrahedron Lett. 2006, 47, 2725.
(a) Simons, M.; Teague, R. M. J. Org. Chem. 1970, 35, 2286. (b) Tanaka, K.; Sugino, T. Green Chem. 2001, 3 133.
(a) Harris, T. M.; Carney, R. L. J. Am. Chem. Soc. 1967, 89, 6734. (b) Hoshino, Y.; Takeno, N. Bull. Chem. Soc. Jpn. 1986, 59, 2903.
Sanicanin, Z.; Tabakovic, I. Tetrahedron Lett. 1986, 27, 407.
Stermitz, F. R.; Adamovics, J. A.; Geigert, J. Tetrahedron 1975, 31, 1593.
Ali, S. M.; Iqbal, J.; Ilyas, M. J. Chem. Res., Synop. 1984, 236.
Saravanamurugan, S.; Palanichamy, M.; Arabindoo, B.; Murugesan, V. Catal. Commun. 2005, 6, 399.
(a) Tokes, A. L.; Szilagyi, L. Synth. Commun. 1987, 17, 1235. (b) Tokes, A. L.; Litkei, G.; Szilagyi, L. Synth. Commun. 1992, 22, 2433.
(a) Varma, R. S.; Saini, R. K. Synlett 1997, 857. (b) Kumar, K. H.; Muralidharan, D.; Perumal, P. T. Synthesis 2004, 63.
(a) Jain, N.; Kumar, A.; Chauhan, S.; Chauhan, S. M. S. Tetrahedron 2005, 61, 1015. (b) Song, C. E. Chem. Commun. 2004, 1033. (c) Binnemans, K. Chem. Rev. 2007, 107, 2592. (d) Wasserscheid, P.; Keim, W. Angew. Chem., Int. Ed. 2000, 39, 3773. (e) Earle, M. J.; Seddon, K. R. Pure Appl. Chem. 2000, 72, 1391. (f) Dupont, J.; de Souza, R. F.; Suarez, P. A. Z. Chem. Rev. 2002, 102, 3667. (g) Welton, T. Chem. Rev. 1999, 99, 2071. (h) Ionic Liquids in Synthesis; Wasserscheid, P.; Welton, T., Eds.; Wiley-VCH: Weinheim, 2002. (i) Zhao, H. Malhotra, S. V. Aldrichim. Acta 2002, 35, 75.
(a) Chippe, C.; Melai, B.; Sanzone, A.; Valentini, G. Pure Appl. Chem. 2009, 81, 2035. (b) Yang, Z.-Z.; He, L.-N.; Dou, X.-Y.; Chanfreau, S. Tetrahedron Lett. 2010, 51, 2931. (c) Xu, D.-Z.; Liu, Y.; Shi, S.; Wang, Y. Tetrahedron: Asymmetry 2010, 21, 2530. (d) Wykes, A.; MacNeil, S. L. Synlett 2007, 107. (e) Dyson, P. J.; Grossel, M. C.; Welton, T. J. Chem. Soc., Dalton Trans. 1997, 3465. (f) Suarez, P. A. Z.; Dupont, J. Polyhedron 1996, 15, 1217. (g) Yang, Z. Z.; He, L. N.; Dou, X. Y.; Chanfreau, S. Tetrahedron Lett. 2010, 51, 2931. (h) Mulik, A.; Chandam, D.; Patil, P.; Patil, D. J. Mol. Liq. 2013, 179, 104. (i) Zare-Bidaki, A.; Davoodnia, A. Bull. Korean Chem. Soc. 2012, 33, 1154.
Pretti, C.; Renzi, M.; Focardi, S. E.; Giovani, A.; Monni, G.; Melai, B.; Rajamani, S.; Chiappe, C. Ecotoxicol. Environ. Saf. 2011, 74, 748.
(a) Kumar, D.; Patel, G.; Kumar, A; Roy, R. K. J. Heterocycl. Chem. 2009, 46, 791. (b) Rao, V. K.; Rao, M. S.; Kumar, A. J. Heterocycl. Chem. 2011, 48, 1356.
(a) Zheng, X.; Jiang, H.; Xie, J.; Yin, Z.; Zhang, H. Synth. Commun. 2013, 43, 1023. (b) Kanagaraj, K.; Pitchumani, K. J. Org. Chem. 2013, 78, 744. (c) Bhattacharya, R. N.; Kundu, P.; Maiti, G. Synth. Commun. 2010, 40, 476. (d) Chandrasekhar, S.; Chatla, S.; Mukhopadhyay, D.; Ganganna, B.; Vijeender, S.; Srihari, P.; Bhadra, U. Bioorg. Med. Chem. Lett. 2012, 22, 645. (e) Xia, Y.; Yang, Z-Y.; Xia, P.; Bastow, K. F.; Tachibana, Y.; Kuo, S-Ch.; Hamel, E.; Hackl, T.; Lee, K-H. J. Med. Chem. 1998, 41, 1155.
Hasaninejad, A.; Shekouhy, M.; Golzar, N.; Zare, A.; Doroodmand, M. M. Appl. Catal., A 2011, 402, 11.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information file to this article containing selected spectral and analytical data of the synthesized compounds is available online at http://link.springer.com/journal/10593.
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(2), 99–103
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2599 kb)
Rights and permissions
About this article
Cite this article
Derabli, C., Mahdjoub, S., Boulcina, R. et al. [C8dabco]Br: a mild and convenient catalyst for intramolecular cyclization of 2-aminochalcones to the corresponding 2-aryl-2,3-dihydroquinolin-4(1H)-ones. Chem Heterocycl Comp 52, 99–103 (2016). https://doi.org/10.1007/s10593-016-1840-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1840-8