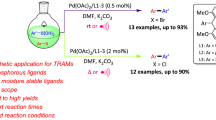
In the current study, five novel perimidinium chlorides were synthesized in good yields by reaction of 1-(3-methylbenzyl)perimidine with five different alkyl chlorides under microwave irradiation in 30 minutes. Synthesized compounds were characterized by IR, 1H, 13C NMR spectroscopic methods and elemental analyses. Performance of in situ formed catalytic system which consist of the perimidinium salts and Pd(OAc)2 was investigated for Heck and Suzuki reactions in aqueous media, and desired products were obtained in good yields.

Similar content being viewed by others
References
Mizoroki, T.; Mori, K.; Ozaki, A. Bull. Chem. Soc. Jpn. 1971, 44, 581.
Heck, R. F.; Nolley, J. P. J. Org. Chem. 1972, 37, 2320.
Miyaura, N.; Yamada, K.; Suzuki, A. Tetrahedron Lett. 1979, 20, 3437.
Beletskaya, I. P.; Cheprakov, A. Chem. Rev. 2000, 100, 3009.
Kotha, S.; Lahiri, K.; Kashinath, D. Tetrahedron 2002, 58, 9633.
Phan, N. T. S.; Van Der Sluys, M.; Jones, C. W. Adv. Synth. Catal. 2006, 348, 609.
Fortman, G. C.; Nolan, S. P. Chem. Soc. Rev. 2011, 40, 5151.
Kovala-Demertzi, D.; Kourkoumelis, N.; Derlat, K.; Michalak, J.; Andreadaki, F. J.; Kostas, I. D. Inorg. Chim. Acta 2008, 361, 1562.
An der Heiden, M.; Plenio, H. Chem.–Eur. J. 2004, 10, 1789.
Martin, R.; Buchwald, S. L. Acc. Chem. Res. 2008, 41, 1461.
Zhou, Z.; Liu, M.; Wu, X.; Yu, H.; Xu, G.; Xie, Y. Appl. Organomet. Chem. 2013, 27, 562.
Sierra, D.; Bhuvanesh, N.; Reibenspies, J. H.; Gladysz, J. A.; Klahn, A. H. J. Organomet. Chem. 2014, 749, 416.
Herrmann, W. A.; Elison, M.; Fischer, J.; Köcher, C.; Artus, G. R. J. Angew. Chem., Int. Ed. 1995, 34, 2371.
Tang, Y.-Q.; Lu, J.-M.; Shao, L.-X. J. Organomet. Chem. 2011, 696, 3741.
Pavia, C.; Ballerini, E.; Bivona, L. A.; Giacalone, F.; Aprile, C.; Vaccaro, L.; Gruttadauria, M. Adv. Synth. Catal. 2013, 355, 2007.
Ozdemir, I.; Yigit, M.; Cetinkaya, E.; Cetinkaya, B. Appl. Organomet. Chem. 2006, 20, 187.
Demir, S.; Zengin, R.; Ozdemir, I. Heteroat. Chem. 2013, 24, 77.
Demir, S.; Ozdemir, I.; Cetinkaya, B. Appl. Organomet. Chem. 2006, 20, 254.
Gulcemal, S.; Kahraman, S.; Daran, J. C.; Cetinkaya, E.; Cetinkaya, B. J. Organomet. Chem. 2009, 694, 3580.
Bazinet, P.; Yap, G. P. A.; Richeson D. S. J. Am. Chem. Soc. 2003, 125, 13314.
Alici, B.; Ozdemir, I.; Karaaslan, K.; Çetinkaya, E.; Cetinkaya, B. J. Mol. Catal. A: Chem. 2005, 231, 261.
Ozdemir, I.; Alici, B.; Gurbuz, N.; Cetinkaya, B.; Çetinkaya E. Russ. J. Coord. Chem. 2005, 31, 142.
Tu, T.; Malineni, J.; Bao, X.; Dötz, K. H. Adv. Synth. Catal. 2009, 351, 1029.
Tsurugi, H.; Fujita, S.; Choi, G.; Yamagata, T.; Ito, S.; Miyasaka, H.; Mashima, K. Organometallics 2010, 29, 4120.
Chou, C.-C.; Yang, C.-C.; Syu, H.-B.; Kuo, T.-S. J. Organomet. Chem. 2013, 745-746, 387.
Arzu Akinci, P.; Gulcemal, S.; Kazheva, O. N.; Alexandrov, G. G.; Dyachenko, O. A.; Cetinkaya, E.; Cetinkaya B. J. Organomet. Chem. 2014, 765, 23.
Bassyouni, F. A.; Abu-Bakr, S. M.; Hegab, K. H.; El-Eraky, W.; El Beih, A. A.; Abdel Rehim, M. E. Res. Chem. Intermed. 2012, 38, 1527.
Azam, M.; Warad, I.; Al-Resayes, S.; Zahin, M.; Ahmad, I.; Shakir, M. Z. Anorg. Allg. Chem. 2012, 638, 881.
Azam, M.; Warad, I.; Al-Resayes, S. I.; Alzaqri, N.; Khan, M. R.; Pallepogu, R.; Dwivedi, S.; Musarrat, J.; Shakir, M. J. Mol. Struct. 2013, 1047, 48.
Chan, A. H.; Wereszczynski, J.; Amer, B. R.; Yi, S. W.; Jung, M. E.; McCammon, J. A.; Clubb, R. T. Chem. Biol. Drug Des. 2013, 82, 418.
Farghaly, T. A.; Mahmoud, H. K. Arch. Pharm. (Weinheim, Ger.) 2013, 346, 392.
Koca, I.; Ungoren, S. H.; Kibriz, I. E.; Yilmaz, F. Dyes Pigm. 2012, 95, 421.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(6), 563–567
Electronic supplementary material
Supplementary information to this article containing 1H and 13C NMR spectra of compounds 1, 2a–e is available for authorized users.
ESM 1
(PDF 644 kb)
Rights and permissions
About this article
Cite this article
Onar, G., Karatas, M.O., Alici, B. et al. Microwave-Assisted Synthesis of Novel Perimidinium Salts as N-Heterocyclic Carbene Precursors: Involvement in Palladium-Catalyzed Cross-Coupling Reactions. Chem Heterocycl Comp 51, 563–567 (2015). https://doi.org/10.1007/s10593-015-1732-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1732-3