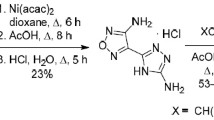
6-Nitroazolo[1,5-а]pyrimidines were used as dipolarophiles in a reaction with sodium azide providing previously unknown 2Н-azolo[1,5-а]-[1,2,3]triazolo[4,5-е]pyrimidines.





Similar content being viewed by others
References
Ugliarolo, E. A.; Gagey, D.; Lantaño, B.; Moltrasio, G. Y.; Campos, R. H.; Cavallaro, L. V.; Moglioni, A. G. Bioorg. Med. Chem. 2012, 20, 5986.
Nieto, M. I.; Caamaño, O.; Fernández, F.; Gómez, M.; Balzarini, J.; De Clercq, E. Nucleosides, Nucleotides Nucleic Acids 2002, 21, 243.
Holý, A.; Dvořáková, H.; Jindřich, J.; Masojídková, M.; Buděšínský, M.; Balzarini, J.; Andrei, G.; De Clercq, E. J. Med. Chem. 1996, 39, 4073.
Diblíková, D.; Kopečná, M.; Školová, B.; Krečmerová, M.; Roh, J.; Hrabálek, A.; Vávrová, K. Pharm. Res. 2014, 31, 1071.
Gigante, A.; Canela, M.-D.; Delang, L.; Priego, E.-M.; Camarasa, M.-J.; Querat, G.; Neyts, J.; Leyssen, P.; Pérez-Pérez, M.-J. J. Med. Chem. 2014, 57, 4000.
Véliz, E. A.; Easterwood, L. H. M.; Beal, P. A. J. Am. Chem. Soc. 2003, 125, 10867.
Gillerman, I.; Fischer, B. J. Med. Chem. 2011, 54, 107.
DeNinno, M. P.; Wright, S. W.; Etienne, J. B.; Olson, T. V.; Rocke, B. N.; Corbett, J. W.; Kung, D. W.; DiRico, K. J.; Andrews, K. M.; Millham, M. L.; Parker, J. C.; Esler, W.; Van Volkenburg, M.; Boyer, D. D.; Houseknecht, K. L.; Doran, S. D. Bioorg. Med. Chem. Lett. 2012, 22, 5721.
Giorgi, I.; Leonardi, M.; Pietra, D.; Biagi, G.; Borghini, A.; Massarelli, I.; Ciampi, O.; Bianucci, A. M. Bioorg. Med. Chem. 2009, 17, 1817.
Gillespie, R. J.; Bamford, S. J.; Botting, R.; Comer, M.; Denny, S.; Gaur, S.; Griffin, M.; Jordan, A. M.; Knight, A. R.; Lerpiniere, J.; Leonardi, S.; Lightowler, S.; McAteer, S.; Merrett, A.; Misra, A.; Padfield, A.; Reece, M.; Saadi, M.; Selwood, D. L.; Stratton, G. C.; Surry, D.; Todd, R.; Tong, X.; Ruston, V.; Upton, R.; Weiss, S. M. J. Med. Chem. 2009, 52, 33.
Giorgi, I.; Bianucci, A. M.; Biagi, G.; Livi, O.; Scartoni, V.; Leonardi, M.; Pietra, D.; Coi, A.; Massarelli, I.; Nofal, F. A.; Fiamingo, F. L.; Anastasi, P.; Giannini, G. Eur. J. Med. Chem. 2007, 42, 1.
Wolkerstorfer, A.; Szolar, O.; Handler, N.; Cusack, S.; Sauvaître, T.; Simon, C.; Morice, C.; Giethlen, B.; Langer, T.; Smith, M.; So, S.-S.; Classen-Houben, D.; Buschmann, H. WO Patent 2013174931.
Babaoglu, K.; Boojamra, C. G.; Eisenberg, E. J.; Hui, H. C.; Mackman, R. L.; Parrish, J. P.; Sangi, M.; Saunders, O. L.; Siegel, D.; Sperandio, D.; Yang, H. WO Patent 2011163518.
Carlson, H. A.; Damm, K. L.; Meagher, K. L. WO Patent 2009036341.
Holden, P. M.; Kaur, H.; Goyal, R.; Gochin, M., Rizzo, R. C. Bioorg. Med. Chem. Lett. 2012, 22, 3011.
Chupakhin, O. N.; Rusinov, V. L.; Ulomskii, E. N.; Charushin, V. N.; Petrov, A. Yu.; Kiselev, O. I. RU Patent 2330036; Byul. Izobret. 2008, (21).
Makisumi, Y. Chem. Pharm. Bull. 1961, 9, 883.
Combs, D.; Langevine, C. M.; Qiu, Y.; Zusi, F. C. WO Patent 2005011609.
Cosyn, L.; Palaniappan, K. K.; Kim, S.-K.; Duong, H. T.; Gao, Z.-G.; Jacobson, K. A.; Van Calenbergh, S. J. Med. Chem. 2006, 49, 7373.
Rusinov, V. L.; Chupakhin, O. N. Nitroazines [in Russian]; Nauka: Novosibirsk, 1991, p. 281.
Rusinov, V. L.; Pilicheva, T. L.; Tumashov, A. A.; Chupakhin, O. N. Chem. Heterocycl. Compd. 1987, 23, 709. [Khim. Geterotsikl. Soedin. 1987, 857.]
Chupakhin, O. N.; Rusinov, V. L.; Pilicheva, T. L.; Tumashov, A. A. Synthesis 1990, 713.
Gorbunov, E. B.; Rusinov, G. L.; Chupakhin, O. N.; Charushin, V. N. Russ. Chem. Bull. 2009, 58, 1309. [Izv. Akad. Nauk, Ser. Khim. 2009, 58, 1272.]
Bastrakov, M. A.; Starosotnikov, A. M.; Pechenkin, S. Yu.; Kachala, V. V.; Glukhov, I. V.; Shevelev, S. A. J. Heterocycl. Chem. 2010, 47, 893.
Kozlowski, K.; Kucybala, Z.; Gaca, J., Jurkowski, R.; Gogolin, R.; Paczkowska, B.; Hyzewicz, K. WO Patent 1985129258.
Rusinov, V. L.; Postovskii, I. Ya.; Petrov, A. Yu.; Sidorov, E. O.; Azev, Yu. A. Chem. Heterocycl. Compd. 1981, 17, 1139. [Khim. Geterotsikl. Soedin. 1981, 1554.]
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
This work was performed with financial support from the Russian Foundation for Basic Research (project 14-03- 31385_mol a) and the Russian Science Foundation (project 14-13-01301).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(5), 491–495
Rights and permissions
About this article
Cite this article
Gorbunov, E.B., Rusinov, G.L., Ulomskii, E.N. et al. Synthesis of 2Н-azolo[1,5-а][1,2,3]triazolo[4,5-е]pyrimidines. Chem Heterocycl Comp 51, 491–495 (2015). https://doi.org/10.1007/s10593-015-1725-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1725-2