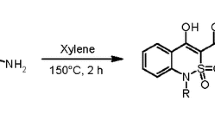
Sodium salt of 3Н -benzo[e][1,3]thiazine-2,4-dione is easily alkylated at position 3. In the case of 2-(arylimino)-2,3-dihydrobenzo[e][1,3]-thiazin-4-ones, the alkylation reaction at this position occurs regioselectively. Alkylation of 2-thioxo-2,3-dihydrobenzo[e][1,3]thiazin-4-one under the same conditions produced regioisomers, but S-alkylation at the exocyclic sulfur atom occurred in the presence of triethylamine. The reaction of 1-(4-oxo-3,4-dihydrobenzo[e][1,3]thiazin-2-ylidene)guanidine with methyl anthranilate led to the formation of tetracyclic quinazolinо[2,1-b]quinazolinedione.
Similar content being viewed by others
References
Trefzer, C.; Rengifo-Gonzalez, M.; Hinner, M. J.; Schneider, P.; Makarov, V.; Cole, S. T.; Johnsson, K. J. Am. Chem. Soc. 2010, 132, 13663.
Tiwari, R.; Moraski, G. C.; Krchňák, V.; Miller, P. A.; Colon-Martinez, M.; Herrero, E.; Oliver, A. G.; Miller, M. J. J. Am. Chem. Soc. 2013, 135, 3539.
Liu, C.-M.; Li, B.; Shen, Y.-H.; Zhang, W.-D. J. Nat. Prod. 2010, 73, 1582.
Solomon, V. R.; Haq, W.; Srivastava, K.; Puri, S. K.; Katti, S. B. J. Med. Chem. 2007, 50, 394.
Kretov, A. E.; Bespalyi, A. S. Zh. Obsch. Khim. 1963, 33, 213.
Takagi, K. Chem. Lett. 1990, 2205.
Capuano, L.; Zander, M. Chem. Ber. 1966, 99, 3085.
Hasspacher, K. US Patent 2978448.
Loev, B.; Kormendy, M. J. Org. Chem. 1962, 27, 3365.
Wentrup, C.; Bender, H.; Gross, G. J. Org. Chem. 1987, 52, 3838.
Lopatina, K. I.; Artemenko, G. N.; Sokolova, T. V.; Avdulov, N. A.; Zagorevskii, V. A. Pharm. Chem. J. 1982, 16, 110. [Khim.-Farm. Zh.. 1982, 16(2), 173.]
Shestakov, A. S.; Prezent, M. A.; Kartsev, V. G.; Shikhaliev, Kh. S. Eur. Chem. Bull. 2014, 3, 713.
Howard, J. C. J. Org. Chem. 1964, 29, 761.
Minkin, V. I.; Olekhnovich, L. P.; Zhdanov Yu. A. Molecular Design of Tautomeric Systems; published by Rostov University: Rostov-on-Don, 1977, p. 17.
Valters, R. E.; Flitsch, W. Ring-chain Tautomerism; Plenum Press: New York, 1985, p. 34.
Butler, K.; Partridge, M. W. J. Chem. Soc. 1959, 1512.
Yun, L. M.; Yangibaev, S.; Shakhidoyatov, Kh. M.; Alekseeva, V. Ya.; V'yunov, K. A. Chem. Heterocycl. Compd. 1987, 23, 214. [Khim. Geterotsikl. Soedin. 1987, 254.]
Bespalov, A. Ya.; Gorchakova, T. L.; Ivanov, A. Yu.; Kuznetsov, M. A.; Kuznetsova, L. M.; Pankova, A. S.; Prokopenko, L. I.; Avdontseva, M. S. Chem. Heterocycl. Compd. 2015, 50, 1547. [Khim. Geterotsikl. Soedin. 2014, 1684.]
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision A.02, Gaussian, Inc.: Wallingford, 2009.
This work was performed with financial support from the Ministry of Education and Science of the Russian Federation, within the framework of State Assgnment for Universities scientific activity for years 2014–2016 (project No. 4.2100.2014/K).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(4), 370–376
Rights and permissions
About this article
Cite this article
Shestakov, A.S., Prezent, M.A., Zlatoustovskaya, E.O. et al. Alkylation of 1,3-benzothiazin-4-one 2-oxo-, 2-arylimino-, and 2-thioxo derivatives. Chem Heterocycl Comp 51, 370–376 (2015). https://doi.org/10.1007/s10593-015-1709-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1709-2