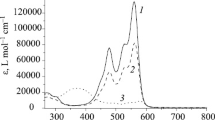
Synthesis of new asymmetric bis-spiropyrans of indoline series based on 6,8-diformyl-5,7-dihydroxy-4-methylcoumarin was realized. Depending on the nature of the substituents in positions 1 and 5 of the indoline moiety the derived compounds may exist in solution in the spiro or merocyanine forms, or as a tautomeric mixture of these forms. UV irradiation of the cyclic forms leads to photocoloring associated with re-opening of one or both of the spiro moieties. The wide variability of spectral kinetic properties of bis-spiropyrans upon fluorescence of photoinduced forms allows one to consider them as molecular switches having absorptive and fluorescent signal functions with the possibility of practical application.




Similar content being viewed by others
Notes
Here and further the name corresponds to the tautomeric form prevalent in CDCl3 solution.
References
Minkin, V. I. Russ. Chem. Rev. 2013, 82, 1. [Usp. Khim. 2013, 82, 1.]
Bertelson, R. C. In Organic Photochromic and Thermochromic Compounds, Crano, J. C.; Guglielmetti, R. J., Eds.; Plenum Press: New York, 1999, vol. 1, p. 11.
Minkin, V. I. Chem. Rev. 2004, 104, 2751.
Guglielmetti, R. In Photochromism: Molecules and Systems, Dürr, H.; Bouas-Laurent, H., Eds.; Elsevier: Amsterdam, 2003, p. 314.
Bercovic, J.; Krongauz, V.; Weiss, V. Chem. Rev. 2000, 100, 1741.
Minkin, V. I. In Molecular Switches, Feringa, B. L.; Browne, W. R., Eds.; Wiley: Weinheim, 2011, p. 37.
Traven, V. F.; Manaev, A. V.; Bochkov, A. Yu.; Chibisova, T. A.; Ivanov, I. V. Russ. Chem. Bull., Int. Ed. 2012, 61, 1342. [Izv. Akad. Nauk SSSR, Ser. Khim. 2012, 1327.]
Traven, V. F.; Miroshnikov, T. A.; Chibisova, T. A.; Barachevsky, V. A.; Venidiktova, O. V.; Strokach, Yu. P. Russ. Chem. Bull., Int. Ed. 2005, 54, 2417. [Izv. Akad. Nauk SSSR, Ser. Khim. 2005, 2342.]
Barachevsky, V. A.; Karpov, R. E.; Venidiktova, O. V.; Valova, T. M.; Strokach, Yu. P.; Miroshnikov, T. A.; Chibisova, T. A.; Traven, V. F. Russ. Chem. Bull., Int. Ed. 2005, 54, 2425. [Izv. Akad. Nauk, Ser. Khim. 2005, 2350.]
Dolotov, S. M.; Miroshnikov, T. A.; Chibisova, T. A.; Sin, S.-L.; Venidiktova, O. V.; Valova, T. M.; Dunaev, A. A.; Strokach, Yu. P.; Barachevsky, V. A.; Traven, V. F. Russ. Chem. Bull., Int. Ed. 2007, 56, 904. [Izv. Akad. Nauk, Ser. Khim. 2007, 870.]
Nikolaeva, O. G.; Tsukanov, A. V.; Shepelenko, E. N.; Lukyanov, B. S.; Metelitsa, A. V.; Kostyrina, O. Yu.; Dubonosov, A. D.; Bren, V. A.; Minkin, V. I. Int. J. Photoenergy 2009, ID 238615, doi: 10.1155/2009/238615.
Nikolaeva, O. G.; Gaeva, E. B.; Shepelenko, E. N.; Tsukanov, A. V.; Metelitsa, A. V.; Lukyanov, B. S.; Dubonosov, A. D.; Bren, V. A.; Minkin, V. I. Russ. J. Org. Chem. 2009, 45, 1091. [Zh. Org. Khim. 2009, 45, 1102.]
Nikolaeva, O. G.; Shepelenko, E. N.; Tsukanov, A. V.; Kozyrev, V. S.; Metelitsa, A. V.; Dubonosov, A. D.; Bren', V. A.; Minkin, V. I. Bull. SSC RAN 2010, 6(3), 12.
Nikolaeva, O. G.; Kostyrina, O. Yu.; Shepelenko, E. N.; Tsukanov, A. V.; Metelitsa, A. V.; Borodkin, G. S.; Dubonosov, A. D.; Bren, V. A.; Minkin, V. I. Russ. J. Org. Chem. 2011, 47, 1370. [Zh. Org. Khim. 2011, 47, 1348.]
Minkin, V. I.; Dubonosov, A. D.; Bren, V. A.; Nikolaeva, O. G.; Tsukanov, A. V.; Burov, O. N.; Fedyanina, A. Yu. Russ. J. Org. Chem. 2013, 49, 374. [Zh. Org. Khim. 2013, 49, 387.]
Adamo, C.; Barone, V. J. Chem. Phys. 1999, 110, 6158.
Barone, V.; Cossi, M. J. Phys. Chem. A 1998, 102, 1995.
Runge, E.; Gross, E. K. U. Phys. Rev. Lett. 1984, 52, 997.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R. Gaussian 03, Revision E.01, 2004.
Pottier, E.; Sergent, M.; Phan Tan Luu, R.; Guglielmetti, R. Bull. Soc. Chim. Fr. 1992, 101, 719.
This work was performed within the framework of implementing the Project part of State Assignment for scientific activity (project № 4.88.2014/K) of the Ministry of Education and Science of the Russian Federation. A. S. Cheprasov, A. V. Metelitsa, and I. V. Dorogan are grateful to the Russian Foundation for Basic Research for financial support (grant 13-03-00901).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(3), 229–223
Rights and permissions
About this article
Cite this article
Nikolaeva, O.G., Karlutova, O.Y., Cheprasov, A.S. et al. Synthesis of bis-spiropyrans based on 6,8-diformyl-5,7-dihydroxy-4-methylcoumarin and photochromic properties thereof. Chem Heterocycl Comp 51, 229–233 (2015). https://doi.org/10.1007/s10593-015-1689-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1689-2