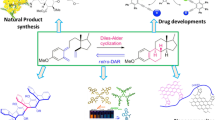
In this research, a green, easy, and effective solvent-free procedure for the synthesis of heterocyclic fullerene derivatives has been achieved via 1,3-dipolar cycloaddition reaction of C60, substituted arylhydrazones/oximes, and PhI(OAc)2 under grinding as a simple physical-mechanical method. This approach does not involve any hazardous organic solvent and has various advantages including design of safe reaction conditions moderate to good yields and short reaction times.

Similar content being viewed by others
References
Kroto, H. W.; Heath, J. R.; Obrien, S. C.; Curl, R. F.; Smalley, R. E. Nature 1985, 318, 162.
Kraetschmer, W.; Lamb, L. D.; Fostiropoulos, K.; Huffman, D. R. Nature 1990, 347, 354.
Hirsch, A.; Brettreich, M. Fullerenes: Chemistry and Reactions; Wiley-VCH: Weinheim, 2005.
Tagmatarchis, N.; Shinohara, H. Mini. Rev. Med. Chem. 2001, 1, 339.
Da Ros, T.; Prato, M. Chem. Commun. 1999, 663.
Jensen, A. W.; Wilson, S. R.; Schuster, D. I. Bioorg. Med. Chem. 1996, 4, 767.
Bakry, R.; Vallant, R. M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Bonn, C. W.; Huck, G. K. Int. J. Nanomed. 2007, 2, 639.
Okumura, M.; Mikawa, M.; Yokawa, T.; Kanazawa, Y.; Kato, H.; Shinohara, H. Acad. Radiol. 2002, 9, 495.
Xing, G. M.; Yuan, H.; He, R.; Gao, X. Y.; Jing, L.; Zhao, F.; Chai, Z. F.; Zhao, Y. L. J. Phys. Chem B. 2008, 112, 6288.
Meng, H.; Xing, G.; Sun, B.; Zhao, F.; Lei, H.; Li, W.; Song, Y.; Chen, Z.; Yuan, H. ; Wang, X.; Long, J.; Chen, C.; Liang, X.; Zhang, N.; Chai, Z.; Zhao, Y. ACS Nano 2010, 4, 2773.
Wudl, F. Acc. Chem. Res. 1992, 25, 157.
Rubin, Y.; Khan, S.; Freedberg, D. I.; Yeretzian, C. J. Am. Chem. Soc. 1992, 115, 344.
Bingel, C. Chem. Ber. 1993, 126, 1957.
Fujiwara, K.; Murata, Y.; Wan, T. S. M.; Komatsu, K. Tetrahedron 1998, 54, 2049.
Mikami, K.; Matsumoto, S.; Ishida, A.; Takamuku, S.; Suenobu, T.; Ukuzumi, S. J. Am. Chem. Soc. 1995, 117, 11134.
Reuther, U.; Hirsch, A. Carbon 2000, 38, 1539.
Forman, G. S.; Tagmatarchis, N.; Shinohara, H. J. Am. Chem. Soc. 2002, 124, 178.
Ishida, H.; Itoh, K.; Ohno M. Tetrahedron 2001, 57, 1737.
Ostrowski, S.; Mikus, A. Mol. Diversity 2003, 6, 315.
Reinov, M. V.; Yurovskaya, M. A.; Streletskiy, A. V.; Boltalina, O. V. Chem. Heterocycl. Compd. 2004, 40, 1150.
Safaei-Ghomi, J.; Masoomi, R. RSC Adv. 2015, DOI: 10.1039/C4RA16020G.
Langa, F.; Nierengarten, J. F. Fullerenes: Principles and Applications; RSC Publishing, 2007.
Yurovskaya, M. A.; Trushkov, I. V. Russ. Chem. Bull., Int. Ed. 2002, 51, 367.
Liu, F.; Du, W.; Liang, Q.; Wang, Y.; Zhang, J.; Zhao, J.; Zhu, S. Tetrahedron 2010, 66, 5467.
Kitamura, H.; Kokubo, K.; Oshima, T. Org. Lett. 2007, 9, 4045.
Ioutsi, V. A.; Zadorin, A. A.; Khavrel, P. A.; Belov, N. M.; Ovchinnikova, N. S.; Goryunkov, A. A.; Kharybin, O. N.; Nikolaev, E. N.; Yurovskaya, M. A.; Sidorov, L. N. Tetrahedron 2010, 66, 3037.
Wang, G. W.; Yang, H. T.; Wu, P.; Miao, C. B.; Xu, Y. J. Org. Chem. 2006, 71, 4346.
Wang, G. W.; Yang, H. T. Tetrahedron Lett. 2007, 48, 4635.
Illescas, B. M.; Martinez-Alvarez, R.; Fernandez-Gadeab, J.; Martin, N. Tetrahedron 2003, 59, 6569.
Li, F. B.; You, X.; Wang, G. W. Org. Lett. 2010, 12, 4896.
Lu, B.; Zhang, J.; Li, J.; Yao, J.; Wang, M.; Zou, Y.; Zhu, S. Tetrahedron 2012, 68, 8924.
Meier, M. S.; Poplawska M. J. Org. Chem. 1993, 58, 4524.
Perez, L.; El-Khouly, M. E.; de la Cruz, P.; Araki, Y.; Ito, O.; Langa, F. Eur. J. Org. Chem. 2007, 2175.
Langa, F.; de la Cruz, P.; Espídora, E.; Gonzalez-Cortes, A.; de la Hoz, A.; Lopez-Arza, V. J. Org. Chem. 2000, 65, 8675.
Modin, J.; Johansson, H.; Grennberg, H. Org. Lett. 2005, 7, 3977.
Irngartinger, H.; Weber, A.; Escher, T. Liebigs. Ann. 1996, 1845.
Yang, H. T.; Ruan, X. J.; Miao, C. B.; Sun, X. Q. Tetrahedron Lett. 2010, 51, 6056.
Langa, F.; de la Cruz, P.; Delgado, J. L.; Gómez-Escalonilla, M. J.; González-Cortés, A.; de la Hoz, A.; López-Arza, V. New J. Chem. 2002, 26, 76.
Anastas, P. T.; Warner, J. C. Green Chemistry: Theory and Practice; Oxford University Press: New York, 1998.
Tanaka, K.; Toda, F. Chem. Rev. 2000, 100, 1025.
Toda, F. Synlett 1993, 303.
Toda, F. Acc. Chem. Res. 1995, 28, 480.
Kumar, S.; Sharma, P.; Kapoor, K. K.; Hundal, M. S. Tetrahedron 2008, 64, 536.
Zohdi, H. F.; Rateb, N. M.; Elnagdy, S. M. Eur. J. Med. Chem. 2011, 46, 5636.
Chen, M.; Wu, J.-L.; Liu, Y.-M.; Cao, Y.; Guo, L.; He, H.-Y.; Fan, K.-N. J. Solid State Chem. 2011, 184, 3357.
Zhou, H.; Tao, K.; Ding, J.; Zhang, Z.; Sun, K.; Shi, W. Colloids Surf., A 2011, 389, 18.
Safaei-Ghomi, J.; Zahedi, S. Monatsh. Chem. 2013, 144, 687.
Wang, S.; He, P.; Zhang, J. M.; Jiang, H.; Zhu, S. Z. Synth. Commun. 2005, 35, 1803.
Safaei-Ghomi, J.; Masoomi, R. RSC Adv. 2014, 4, 2954.
Matsubara, Y.; Tada, H.; Nagase, S.; Yoshida, Z. J. Org. Chem. 1995, 60, 5372.
Hossain, M. D.; Kitamura, T. Tetrahedron Lett. 2006, 47, 7889.
Furniss, B. S.; Hannaford, A. J.; Smith, P. W. G.; Tatchell, A. R. Vogel's Textbook of Practical Organic Chemistry; Pearson Education, Dorling Kindersley, 2008, 5th ed., p. 900–1050.
The authors are grateful to University of Kashan for supporting this work by Grant No. 159196/XXI.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(1), 39–43
Rights and permissions
About this article
Cite this article
Safaei-Ghomi, J., Masoomi, R. Grinding-induced synthesis of heterocyclic fullerene derivatives under solvent-free conditions. Chem Heterocycl Comp 51, 39–43 (2015). https://doi.org/10.1007/s10593-015-1657-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1657-x