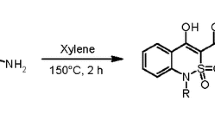
The alkylation of methyl 4-hydroxy-1-methyl-2,2-dioxo-1Н-2λ6,1-benzothiazine-3-carboxylate in DMSO or acetone with ethyl iodide at 25°С gave a mixture of 3-ethyl- and 4-ethoxy-substituted derivatives, in complete agreement with quantum-chemical calculations. The ratio of С- and О-alkylation products changed depending on the alkali metal carbonate used as a base. The reaction practically did not proceed in anhydrous THF or water due to the low nucleophilicity of the anion.


Similar content being viewed by others
References
I. V. Ukrainets, L. A. Petrushova, N. L. Bereznyakova, and Liu Yangyang, Chem. Heterocycl. Compd., 50, 1346 (2014). [Khim. Geterotsikl. Soedin., 1459 (2014).]
A. F. Pozharskii, Theoretical Foundations of Heterocyclic Chemistry [in Russian], Khimiya, Moscow (1985).
V. A. Shchadrikova, E. V. Golovin, and Yu. N. Klimochkin, Chem. Heterocycl. Compd., 50, 1586 (2014). [Khim. Geterotsikl. Soedin., 1725 (2014).]
B. Baghernejad, Eur. J. Chem., 1, 54 (2010).
V. V. Sureshbabu and N. Narendra, in: A. B. Hughes (editor), Amino Acids, Peptides, and Proteins in Organic Chemistry, Vol. 4, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim (2011), p. 1.
M. Schelhaas and H. Waldmann, Angew. Chem., Int. Ed., 35, 2056 (1996).
T. W. Greene and P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley-Interscience, New York (1999).
F. A. Carey and R. J. Sundberg, Advanced Organic Chemistry. Part B: Reactions and Synthesis, Springer (2007), p. 215.
S. G. Kuznetsov, S. M. Chigareva, and S. M. Ramsh, Itogi Nauki i Tekhniki. VINITI. Ser. Org. Khim., 19, 25 (1991).
A. R. Wohl, A. R. Michel, S. Kalscheuer, C. W. Macosko, J. Panyam, and T. R. Hoye, J. Med. Chem., 57, 2368 (2014).
C. B. Bourguet, P.-L. Boulay, A. Claing, and W. D. Lubell, Bioorg. Med. Chem. Lett., 24, 3361 (2014).
G. Audran, P. Brémond, J.-M. Franconi, S. R. A. Marque, P. Massot, P. Mellet, E. Parzy, and E. Thiaudière, Org. Biomol. Chem., 12, 719 (2014).
A. M. Qandil, Int. J. Mol. Sci., 13, 17244 (2012).
I. V. Ukrainets, L. A. Petrushova, and S. P. Dzyubenko, Chem. Heterocycl. Compd., 49, 1378 (2013). [Khim. Geterotsikl. Soedin., 1479 (2013).]
I. V. Ukrainets, S. G. Taran, S. V. Gorokhova, I. V. Gorlacheva, P. A. Bezugly, and A. V. Turov, Chem. Heterocycl. Compd., 32, 952 (1996). [Khim. Geterotsikl. Soedin., 1104 (1996)].
S. V. Shishkina, I. V. Ukrainets, and L. A. Petrushova, Acta Crystallogr., Sect. E: Struct. Rep. Online, E70, o786 (2014).
B. Loev, M. F. Kormendy, and K. M. Snader, J. Org. Chem., 31, 3531 (1966).
F. T. Coppo and M. M. Fawzi, J. Heterocycl. Chem., 35, 983 (1998).
I. V. Ukrainets, L. A. Petrushova, S. P. Dzyubenko, and Liu Yangyang, Chem. Heterocycl. Compd., 50, 1047 (2014). [Khim. Geterotsikl. Soedin., 1135 (2014).]
N. S. Zefirov, V. A. Palyulin, and E. E. Dashevskaya, J. Phys. Org. Chem., 3, 147 (1990).
Yu. V. Zefirov, Crystallogr. Rep., 42, 865 (1997). [Kristallografiya, 42, 936 (1997).]
A. G. Orpen, L. Brammer, F. H. Allen, O. Kennard, D. G. Watson, and R. Taylor, in: B. Burgi and J. D. Dunitz (editors), Structure Сorrelation, Vol. 2, H.- VCH, Weinheim (1994), p. 741.
R. G. Parr and W. Yang, Density Functional Theory of Atoms and Molecules, Oxford University Press, New York (1989).
Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 120, 215 (2008).
F. Weinhold, in: P. v. R. Schleyer, N. L. Allinger, T. Clark, J. Gasteiger, P. A. Kollman, H. F. Schaefer III, and P. R. Schreiner (editors), Encyclopedia of Computational Chemistry, Vol. 3, John Wiley & Sons, Chichester (1998), p. 1792.
H. Bekker, G. Domshke, E. Fanghenel, M. Fischer, K. Gevald, R. Mayer, D. Pafel, G. Schmidt, K. Shvetlik, V. Berger, I. Faust, F. Gentz, R. Glukh, K. Mueller, K. Schollberg, E. Zeiler, and G. Zeppenfeld, Organicum [Russian translation], Vol. 1, Mir, Moscow (1992), p. 205.
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., A64, 112 (2008).
The authors would like to express their gratitude to Senior Researcher F. M. Dolgushin (A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Moscow) for assistance with X-ray structural analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
*For Communication 8, see [1].
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 12, pp. 1892-1899, December, 2014.
Rights and permissions
About this article
Cite this article
Ukrainets, I.V., Petrushova, L.A., Shishkina, S.V. et al. 2,1-Benzothiazine 2,2-Dioxides. 9*. Alkylation of Methyl 4-Hydroxy-1-Methyl-2,2-Dioxo-1Н-2λ6,1-Benzothiazine-3-Carboxylate with Ethyl Iodide. Chem Heterocycl Comp 50, 1741–1747 (2015). https://doi.org/10.1007/s10593-015-1646-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1646-0