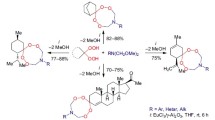
Alkylaromatic α-hydroxylamino ketones with a p-hydroxy(alkoxy)aryl substituent were used for the preparation of stable diastereomeric spirocyclic nitroxyl radicals of 3-imidazoline series, having two different or identical mesogenic groups in the molecule. The molecular structure of these compounds was determined by NMR study of their diamagnetic reduced derivatives.



Similar content being viewed by others
References
P. M. Lahti (editor), Magnetic Properties of Organic Materials, Marcel Dekker, New York (1999), p. 728.
N. Ikuma, R. Tamura, S. Shimono, N. Kawame, O. Tamada, N. Sakai, J. Yamauchi, and Y. Yamamoto, Angew. Chem., Int. Ed., 43, 3677 (2004).
R. Tamura, Y. Uchida, and N. Ikuma, J. Mater. Chem., 18, 2872 (2008).
R. Tamura, Y. Uchida, and K. Suzuki, in: J. W. Goodby, P. J. Collings, T. Kato, C. Tschierske, H. F. Gleeson, and P. Raynes (editors), Handbook of Liquid Crystals: 8 Volume Set, Vol. 8, Wiley-VCH, Weinheim (2014), p. 837.
R. Tamura, Y. Uchida, and K. Suzuki, in: Q. Li (editor), Liquid Crystals Beyond Display: Chemistry, Physics, and Applications, J. Wiley & Sons, Inc., Hoboken (2012), p. 83.
R. Tamura, Y. Uchida, and K. Suzuki, in: A. I. Kokorin (editor), Nitroxides – Theory, Experiment and Applications, InTech, Rijeka (2012), p. 191.
Y. Uchida, N. Ikuma, R. Tamura, S. Shimono, Y. Noda, J. Yamauchi, Y. Aoki, and H. Nohira, J. Mater. Chem., 18, 2950 (2008).
Y. Uchida, K. Suzuki, R. Tamura, N. Ikuma, S. Shimono, Y. Noda, and J. Yamauchi, J. Am. Chem. Soc., 132, 9746 (2010).
R. Kogo, F. Araoka, Y. Uchida, R. Tamura, K. Ishikawa, and H. Takezoe, Appl. Phys. Express, 3, 041701 (2010).
K. Suzuki, Y. Uchida, R. Tamura, Y. Noda, N. Ikuma, S. Shimono, and J. Yamauchi, Soft Matter, 9, 4687 (2013).
W. Schmidt, F. Vögtle, and E. Poetsch, Liebigs Ann., 1319 (1995).
W. Calaminus, F. Vögtle, and R. Eidenschink, Z. Naturforsch., B: Anorg. Chem., Org. Chem., 41B, 1011 (1986).
J. Boettcher, R. Hartmann, and F. Vögtle, Chem. Ber., 125, 1865 (1992).
E. Poetsch, W. Schmidt, F. Vögtle, and N. Feuerbacher, DE Pat. Appl. 19755245; Chem. Abstr., 131, P65963j (1999).
N. Feuerbacher, F. Vögtle, J. Windscheidt, E. Poetsch, and M. Nieger, Synthesis, 117 (1999).
K. Miyazawa, D. S. Yufit, J. A. K. Howard, and A. de Meijere, Eur. J. Org. Chem., 4109 (2000).
K. Miyazawa, D. Demus, and A. de Meijere, Mol. Cryst. Liq. Cryst., 364, 253 (2001).
T. Itoh, M. Kanbara, S. Nakajima, Y. Sakuta, S. Hayase, M. Kawatsura, T. Kato, K. Miyazawa, and H. Uno, J. Fluorine Chem., 130, 1157 (2009).
Q. Cui and R. P. Lemieux, J. Mater. Chem.C, 1, 1011 (2013).
Q. Cui and R. P. Lemieux, Liq. Cryst., 40, 1609 (2013).
Y. Uchida, N. Matsuoka, H. Takahashi, S. Shimono, N. Ikuma, and R. Tamura, Heterocycles, 74, 607 (2007).
K. Suzuki, D. G. Mazhukin, H. Takahashi, Y. Uchida, R. Tamura, and I. A. Grigor’ev, Heterocycles, 78, 3091 (2009).
E. V. Zaitseva, Yu. V. Gatilov, S. A. Amitina, R. Tamura, I. A. Grigor’ev, and D. G. Mazhukin, Zh. Org. Khim, 50, 78 (2014). [Russ. J. Org. Chem., 50, 72 (2014).]
R. Tamura, K. Suzuki, Y. Uchida, and Y. Noda, in: B. C. Gilbert, D. M. Murphy, and V. Chechik (editors), Electron Paramagnetic Resonance, Vol. 23, RSC Publishing (2013), p.1.
T. K. Sevast’yanova and L. B. Volodarskii, Izv. Akad. Nauk SSSR, Ser. Khim., 2339 (1972). [Bull. Acad. Sci. USSR, Div. Chem. Sci., 21, 2276 (1972).]
V. A. Reznikov and L. B. Volodarskii, Khim. Geterotsikl. Soedin., 772 (1990). [Chem. Heterocycl. Compd., 643 (1990).]
F. Hintermaier, L. B. Volodarsky, K. Polborn, and W. Beck, Liebigs Ann., 2189 (1995).
V. A. Reznikov and L. B. Volodarskii, Izv. Akad. Nauk SSSR, Ser. Khim., 1654 (1997). [Russ. Chem. Bull.), 46, 1577 (1997).]
L. B. Volodarsky, in: L. B. Volodarsky (editor), Imidazoline Nitroxides: Synthesis and properties, Vol. 1, CRC Press, Boca Raton (1988), p. 232.
M. Haslanger and R. G. Lawton, Synth. Commun., 4, 155 (1974).
K. C. Kumara Swamy, N. N. Bhuvan Kumar, E. Balaraman, and K. V. P. Pavan Kumar, Chem. Rev., 109, 2551 (2009).
http://limor1.nioch.nsc.ru/quant/conformers/shern/spiroimidazol/.
F. R. Jensen, C. H. Bushweller, and B. H. Beck, J. Am. Chem. Soc., 91, 344 (1969).
H.-J. Schneider and V. Hoppen, J. Org. Chem., 43, 3866 (1978).
G. Impre, I. Jakli, A. Kalaszi, and O. Farkas, Advanced Automatic Generation of 3D Molecular Structures, 1st European Chemistry Congress, Budapest, Hungary, 27-31 August, 2006.
C.-E. Chang and M. K. Gilson, J. Comput. Chem, 24, 1987 (2003).
J. P. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett., 77, 3865 (1996).
D. N. Laikov, Chem. Phys. Lett., 416, 116 (2005).
L. B. Volodarskii, A. S. Lapik, V. V. Russkikh, V. S. Kobrin, E. F. Lavretskaya, L. I. Volkova, D. A. Sarkisyan, and M. M. Borisov, USSR Inventor’s Certificate 657016; Chem. Abstr., 91, P68767q (1979).
U. B. Vasconcelos, E. Dalmolin, and A. A. Merlo, Org. Lett., 7, 1027 (2005).
This work was done owing financial support from the Russian Foundation for Basic Research – JSPS (Japanese Society for the Promotion of Science) (project No.11-03-92107-YaF-а), Integration program of the Siberian Branch, Russian Academy of Sciences (Cooperative Basic Research, project No. 1), and the Ministry of Education and Science of the Russian Federation (contract 8456).
The spectral investigations, elemental analysis, and differential scanning calorimetry were performed at the Certified Testing Analytical Center of the Novosibirsk Institute of Organic Chemistry, Siberian Branch of the Russian Academy of Sciences (Novosibirsk).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1207-1220, August, 2014.
Rights and permissions
About this article
Cite this article
Zaitseva, E.V., Shernyukov, A.V., Amitina, S.A. et al. Synthesis of Diastereomeric Spirocyclic Nitroxyl Radicals of 3-Imidazoline Series with Two Mesogenic Groups. Chem Heterocycl Comp 50, 1113–1125 (2014). https://doi.org/10.1007/s10593-014-1571-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1571-7