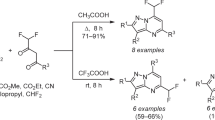
We have optimized the reaction conditions for the synthesis of 2-methylsulfanyl-6-polyfluoro-alkylpyrimidin-4-ones. In particular the yield of trifluoromethyl-substituted heterocycle was increased to 96%, and new polyfluoroalkyl-substituted analogs were prepared. The interaction of these heterocycles with morpholine or hydrazine was shown to result in nucleophilic substitution of the methylsulfanyl group, leading to 2-morpholino- and 2-hydrazino-6-polyfluoroalkylpyrimidin-4-ones. The reaction of 2-(2-phenylhydrazino)-6-trifluoromethylpyrimidin-4-one with paraform produced 5-oxo-2-phenyl-7-tri-fluoromethyl-5H-1,2,4-triazolo[4,3-а]pyrimidin-1-id-2-ium. The lactam structure of the synthesized heterocycles was established by X-ray structural analysis, as well as by IR and NMR spectroscopy.




Similar content being viewed by others
References
H. Nakamura, J. Y. Noh, K. Itoh, S. Fukata, A. Miyauchi, and N. Hamada, J. Clin. Endocrinol. Metab., 92, 2157 (2007).
S. Prachayasittikul, N. Sornsongkhram, R. Pingaew, S. Techatanachai, S. Ruchirawat, and V. Prachayasittikul, Eur. J. Sci. Res., 36, 236 (2009).
M. B. Nawrozkij, D. Rotili, D. Tarantino, G. Botta, A. S. Eremiychuk, I. Musmuca, R. Ragno, A. Samuele, S. Zanoli, M. Armand-Ugón, I. Clotet-Codina, I. A. Novakov, B. S. Orlinson, G. Maga, J. A. Esté, M. Artico, and A. Mai, J. Med. Chem., 51, 4641 (2008).
M. T. Abdel-Aal, Arch. Pharm. Res., 33, 797 (2010).
H. Gershon, D. D. Clarke, A. T. Grefig, and T. E. Anderson, Monatsh. Chem., 121, 289 (1990).
A. A.-H. Abdel-Rahman, A.-A. SH. El-Etrawy, A. E.-S. Abdel-Megied, I. F. Zeid, and El S. H. El Ashry, Nucleosides, Nucleotides Nucleic Acids, 27, 1257 (2008).
M. Radi, E. Petricci, F. Corelli, and M. Botta, Heterocycles, 72, 79 (2007).
A. A.-H. Abdel-Rahman, and M. T. Abdel-Aal, J. Chem. Res., Synop., 251 (2002).
V. Syadyaryavichyute and P. Vainilavichyus, Khim. Geterotsikl. Soedin., 1525 (1992). [Chem. Heterocycl. Compd., 28, 1304 (1992).]
H. R. Bizzo, O. A. C. Antunes, and A. L. Gemal, Heterocycl. Commun., 9, 359 (2003).
P. I. Vainilavichyus and V. Syadyaryavichyute, Khim. Geterotsikl. Soedin., 1655 (1987). [Chem. Heterocycl. Compd., 23, 1332 (1987).]
A. I. Rakhimov, I. Yu. Kameneva, M. B. Nawrozkij, E. S. Titova, and S. V. Kudashev, Zh. Obshch. Khim., 78, 828 (2008). [Russ. J. Gen. Chem., 78, 971 (2008).]
M. M. Heravi and R. Motamedi, Heterocycl. Commun., 11, 19 (2005).
M. R. Shaaban, H. Ishii, and T. Fuchigami, J. Org. Chem., 65, 8685 (2000).
A. V. Erkin, V. I. Krutikov, and M. A. Chubraev, Zh. Obshch. Khim., 74, 466 (2004). [Russ. J. Gen. Chem., 74, 423 (2004).]
B. Tait, N. A. Powell, and M. Cullen, WO Pat. Appl. 2012154880.
B. Abarca, С. Soriano, and G. Jones, J. Chem. Res., Synop., 158 (1987).
V. S. Reznik, I. Sh. Salikhov, Yu. S. Shvetsov, Yu. Ya. Efremov, and I. Kh. Rizvanov, Izv. Akad. Nauk, Ser. Khim., 335 (1995). [Russ. Chem. Bull., 44, 326 (1995).]
V. A. Yanchenko, A. N. Gur’eva, A. R. Khairulin, and A. M. Demchenko, Khim. Geterotsikl. Soedin., 1296 (2002). [Chem. Heterocycl. Compd., 38, 1138 (2002).]
R. Ringom, E. Axen, J. Uppenberg, T. Lundbäck, L. Rondahl, and T. Barf, Bioorg. Med. Chem. Lett., 14, 4449 (2004).
M. L. Maddess and R. Carter, Synthesis, 44, 1109 (2012).
Y. Nakagawa, S. Bobrov, C. R. Semer, T. A. Kucharek, and M. Hamamoto, US Pat. Appl. 20050038041.
H. Mizuno, WO Pat. Appl. 2010134478.
R. Domori, Y. Tanaka, and S. Miyazaki, JP Pat. Appl. 42014952. Сhem. Аbstr., 68, 105224 (1968).
H. Gershon, A. T. Grefig, and A. A. Scala, J. Heterocycl. Chem., 20, 219 (1983).
G. Vasilev, N. Spasovska, A. Spasov, and G. Kimenov, Dokl. Bolg. Akad. Nauk, 32, 809 (1979); Сhem. Аbstr., 92, 53262 (1980).
R. Pelova, N. Spassowska, L. Maneva, and S. Taxirov, Pharmazie, 42, 251 (1987).
A. S. Shawali, R. H. Hilal, and S. El-Sheikh, Monatsh. Chem., 132, 715 (2001).
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., A64, 112 (2008).
The work was performed with financial support from the Russian Foundation for Basic Research (grant 13-03-00617) and the Grants Council of the President of the Russian Federation (grant NSh-3656.2014.3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 6, pp. 928-935, June, 2014.
Rights and permissions
About this article
Cite this article
Khudina, O.G., Burgart, Y.V. & Saloutin, V.I. 2-Methylsulfanyl-6-Polyfluoroalkyl-Pyrimidin-4-Ones: Synthesis and Nucleophilic Substitution Reactions. Chem Heterocycl Comp 50, 856–863 (2014). https://doi.org/10.1007/s10593-014-1539-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1539-7