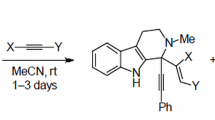
We optimized the reaction of tetrahydropyridine ring expansion in 1-aryl-substituted tetrahydro-β-carbolines by the action of activated alkynes and achieved higher than 70% yields of the target indoloazocines. The substituents in the 1-aryl ring and at the indole nitrogen atom were shown to affect the rate and selectivity of this transformation.
Similar content being viewed by others
References
C. Caron, A. Graftieaux, G. Massiot, L. Le Men-Olivier, and C. Delaude, Phytochemistry, 28, 1241 (1989).
M. Zeches, T. Ravao, B. Richard, G. Massiot, L. Le Men-Olivier, and R. Verpoorte, J. Natural Products, 50, 714 (1987).
L. G. Voskressensky, L. N. Kulikova, T. N. Borisova, and A. V. Varlamov, Adv. Heterocycl. Chem., 96, 81 (2008).
L. G. Voskressensky, T. N. Borisova, L. N. Kulikova, A. V. Varlamov, M. Catto, C. Altomare, and A. Carotti, Eur. J. Org. Chem., 3128 (2004).
L. G. Voskressensky, T. N. Borisova, L. N. Kulikova, E. G. Dolgova, A. I. Kleimenov, E. A. Sorokina, A. A. Titov, and A. V. Varlamov, Khim. Geterotsikl. Soedin., 703 (2007). [Chem. Heterocycl. Compd., 43, 587 (2007).]
L. G. Voskressensky, L. N. Kulikova, A. S. Kasatochkina, A. V. Listratova, F. A. Toze, T. N. Borisova, and A. V. Varlamov, Khim. Geterotsikl. Soedin., 1253 (2010). [Chem. Heterocycl. Compd., 46, 1013 (2010).]
A. S. Dneprovskii and T. N. Temnikova, Theoretical Foundations of Organic Chemistry [in Russian], Khimiya, Leningrad (1991), p. 86.
Á. González-Gómez, G. Domínguez, U. Amador, and J. Pérez-Castells, Tetrahedron Lett., 49, 5467 (2008).
L. G. Voskressensky, T. N. Borisova, A. A. Titov, A. V. Listratova, L. N. Kulikova, A. V. Varlamov, V. N. Khrustalev, and G. G. Aleksandrov, Izv. Akad. Nauk, Ser. Khim., 6, 1218 (2012). [Russ. Chem. Bull., Int. Ed., 61, 1231 (2012).]
The work received support from the Russian Foundation for Basic Research (grants 14-03-0031 and 13-03-90431 Ukr_f_a).
The authors would like to express their gratitude to the Collective Use Center for Physical and Chemical Investigations of the Peoples’ Friendship University for performing elemental analyses and recording the IR spectra.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 5, pp. 716-728, May, 2014.
Rights and permissions
About this article
Cite this article
Voskressensky, L.G., Borisova, T.N., Chervyakova, T.M. et al. Synthesis of 6-aryl-Substituted Azocino-[5,4-b]indoles from 1-aryl-Substituted 2-Ethyltetrahydro-β-Carbolines. Chem Heterocycl Comp 50, 658–669 (2014). https://doi.org/10.1007/s10593-014-1518-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1518-z