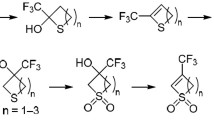
We have developed a thionylation – hetero-Diels–Alder domino reaction using α,β-unsaturated ketones with 1,2,3-triazole substituents, namely, 3-[2-(allyloxy)phenyl]-1-(1-aryl-5-methyl-1H-1,2,3-triazol-4-yl)-prop-2-en-1-ones, leading to 3,4-dihydro-2Н-thiopyranes and thiopyrano[3,4-с]chromenones. Variants of intra- and intermolecular cycloaddition were studied, as well the stereo- and regioselectivity of such reactions was assessed.
Similar content being viewed by others
References
J. D. Hepworth and B. M. Heron, in: A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven, and R. J. K. Taylor (editors), Comprehensive Heterocyclic Chemistry III, Vol. 7, Elsevier, Amsterdam (2008), p. 727.
L. I. Markova and V. G. Kharchenko, Khim. Geterotsikl. Soedin., 613 (1994). [Chem. Heterocycl. Compd., 30, 537 (1994).]
V. D. Dyachenko, S. G. Krivokolysko, and V. P. Litvinov, Khim. Geterotsikl. Soedin., 1099 (1996). [Chem. Heterocycl. Compd., 32, 947 (1996).]
L. V. Timokhina, O. V. Sokol'nikova, L. V. Kanitskaya, D.-S. D. Toryashinova, and M. G. Voronkov, Khim. Geterotsikl. Soedin., 1697 (2005). [Chem. Heterocycl. Compd., 41, 1430 (2005).]
L. F. Tietze, G. Brasche, and K. Gericke, Domino Reactions in Organic Synthesis, Wiley-VCH, Weinheim (2006), p. 297.
L. F. Tietze and N. Rackelmann, Pure Appl. Chem., 76, 1967 (2004).
L. F. Tietze, Chem. Rev., 96, 115 (1996).
F. M. Moghaddam, M. Kiamehr, S. Taheri, and Z. Mirjafary, Helv. Chim. Acta, 93, 964 (2010).
K. C. Majumdar, A. Taher, and K. Ray, Tetrahedron Lett., 50, 3889 (2009).
V. S. Matiychuk, R. B. Lesyk, M. D. Obushak, A. Gzella, D. V. Atamanyuk, Y. V. Ostapiuk, and A. P. Kryshchyshyn, Tetrahedron Lett., 49, 4648 (2008).
A. O. Bryhas, Y. I. Horak, Y. V. Ostapiuk, M. D. Obushak, and V. S. Matiychuk, Tetrahedron Lett., 52, 2324 (2011).
E. Ceulemans, M. Voets, S. Emmers, K. Uytterhoeven, L. V. Meervelt, and W. Dehaen, Tetrahedron, 58, 531 (2002).
E. Ceulemans, M. Voets, S. Emmers, and W. Dehaen, Synlett, 1155 (1997).
T. Saito, H. Furuie, Y. Ishigo-oka, I. Watanabe, and K. Kobayashi, Heterocycles, 53, 1685 (2000).
T. Saito, H. Kimura, K. Sakamaki, T. Karakasa, and S. Moriyama, J. Chem. Soc., Chem. Commun., 811 (1996).
T. Saito, M. Nagashima, T. Karakasa, and S. Motoki, J. Chem. Soc., Chem. Commun., 411 (1992).
T. Saito, M. Nagashima, T. Karakasa, and S. Motoki, J. Chem. Soc., Chem. Commun., 1665 (1990).
I. T. Barnish, C. W. G. Fishwick, and D. R. Hill, Tetrahedron Lett., 32, 405 (1991).
N. T. Pokhodylo, R. D. Savka, V. S. Matiychuk, and N. D. Obushak, Zh. Obsch. Khim., 79, 320 (2009). [Russ. J. Gen. Chem., 79, 309 (2009).]
H.-S. Dong, H.-C. Wang, Z.-L. Gao, R.-S. Li, and F.-H. Cui, J. Heterocycl. Chem., 47, 389 (2010).
The project received financial support from the State Fund of Fundametntal Research of Ukraine (project F53.3/013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 4, pp. 593-598, April, 2014.
Rights and permissions
About this article
Cite this article
Pokhodylo, N.T., Savka, R.D. & Obushak, M.D. Synthesis of 3,4-Dihydro-2Н-Thiopyrans and Thiopyrano[3,4-с]Chromenes Having a 1,2,3-Triazole Substituent by Using Thionylation – Hetero-Diels–Alder Domino Reaction. Chem Heterocycl Comp 50, 544–549 (2014). https://doi.org/10.1007/s10593-014-1505-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1505-4