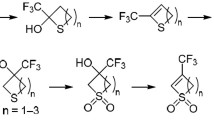
2-(p-Tolylsulfanyl)-2-trifluoromethyl-3,6-dihydro-2Н-thiopyran 1-oxides undergo a vinylogous Pummerer reaction upon interaction with trifluoroacetic anhydride, forming 2-(p-tolylsulfanyl)-4-tri-fluoroacetoxy-2-trifluoromethyl-3,4-dihydro-2Н-thiopyrans. The hydrolysis, acetylation, and free radical desulfanylation of these compounds with a subsequent oxidation of the sulfur atom leads to 4-acetoxy-2-trifluoromethyl-3,4-dihydro-2Н-thiopyran 1-oxides. The Pummerer addition reaction of the latter with acetic anhydride and boron trifluoride etherate results in a ring contraction and formation of 3-acet-oxy-2-diacetoxymethyl-5-(trifluoromethyl)thiolanes, which could be converted with sodium boro-hydride to 3-hydroxy-2-hydroxymethyl-5-(trifluoromethyl)thiolanes having antiviral activity.

Similar content being viewed by others

References
K. S. Feldman, Tetrahedron, 62, 5003 (2006).
L. H. S. Smith, S. C. Coote, H. F. Sneddon, and D. J. Procter, Angew. Chem., Int. Ed., 49, 5832 (2010).
F. Santoyo Gonzalez, P. Garcia Mendoza, and F. J. Lopez Apricio, Carbohydr. Res., 183, 227 (1988).
J. Fujita, H. Matsuda, K. Yamamoto, Y. Morii, M. Hashimoto, T. Okuno, and K. Hashimoto, Tetrahedron, 60, 6829 (2004).
Y. Watanabe and T. Sakakibara, Tetrahedron, 65, 599 (2009).
Y. Yoshimura, Y. Yamazaki, Y. Saito, and H. Takahata, Tetrahedron, 65, 9091 (2009).
S. A. Siry and V. M. Timoshenko, Tetrahedron Lett., 52, 6260 (2011).
S. A. Siry and V. M. Timoshenko, Tetrahedron Lett., 51, 6406 (2010).
A. Yu. Sizov, A. N. Kovregin, R. N. Serdyuk, M. V. Vorob'ov, V. A. Porosyatnikov, A. A. Tsvetkov, D. O. Korneev, and A. F. Yermolov, Izv. Akad. Nauk, Ser. Khim., 1156 (2006). [Russ. Chem. Bull., 55, 1200 (2006).]
V. M. Timoshenko, S. A. Siry, A. B. Rozhenko, and Yu. G. Shermolovich, J. Fluorine Chem., 131, 172 (2010).
M. Denancé, R. Legay, A.-C. Gaumont, and M. Gulea, Tetrahedron Lett., 49, 4329 (2008).
W. L. Dolbier, Jr., Guide to Fluorine NMR for Organic Chemists, John Wiley & Sons, Hoboken (2009), p. 140.
A. N. Alexeenko and V. P. Nazaretian, J. Fluorine Chem., 69, 241 (1994).
M. Heras, M. Gulea, S. Masson, and C. Phillouze, Eur. J. Org. Chem., 160 (2004).
Yu. V. Zefirov and P. M. Zorkii, Usp. Khim., 64, 446 (1995).
E. Juáres, A. García, H. Hommer, M. Salas, and B. Gordillo, Heteroat. Chem., 17, 289 (2006).
N. A. Hughes, K.-M. Kuhajda, and D. A. Miljkovic, Carbohydr. Res., 257, 299 (1994).
I. Robina, P. Vogel, and Z. Witczak, Curr. Org. Chem., 5, 1177 (2001).
H. Ikehira and S. Tanimoto, Bull. Chem. Soc. Jpn., 57, 2474 (1984).
O. Hromatka, D. Binder, and K. Eichinger, Monatsh. Chem., 105, 127 (1974).
D. Binder, C. R. Noe, K. Baumann, and J. M. F. Wildburger, Arch. Pharm., 318, 243 (1985).
С. G. Krespan, J. Org. Chem. 27, 3588 (1962).
T. Abe, S. Nagase, and H. Baba, Bull. Chem. Soc. Jpn., 46, 3845 (1973).
R. N. Renaud and P. J. Champagne, M. Savard, Can. J. Chem., 57, 2617 (1979).
W. Dmovski and T. Kozłovski, J. Fluorine Chem., 87, 179 (1998).
F. Neese, ORCA – an ab initio, Density Functional and Semiempirical Program Package, Version 2.7, University of Bonn (2009).
F. Neese, J. Co and mp. Chem., 24, 1740 (2003).
R. Ahlrichs, M. Bär, M. Häser, H. Horn, and C. Kölmel, Chem. Phys. Lett., 162, 165 (1989).
A. Schäfer, C. Huber, and R. Ahlrichs, J. Chem. Phys., 100, 5829 (1994).
S. Sinneker, A. Rajendran, A. Klamt, M. Diedenhofen, and F. Neese, J. Phys. Chem. A, 110, 2235 (2006).
D. J. Watkin, C. K. Prout, J. R. Carruthers, and P. W. Betteridge, CRYSTALS, Issue 10, Chemical Crystallography Laboratory, University of Oxford, (1996).
J. R. Carruthers and D. J. Watkin, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., A35, 698 (1979).
M. V. Berridge, P. M. Herst, and A. S. Tan, Biotechnol. Annu. Rev., 11, 127 (2005).
S. D. Zagorodnya and N. V. Nesterova, Mikrobiol. Zh., 73, No. 2, 65 (2011).
A. M. Shcherbinskaya, N. S. Dyachenko, S. L. Rybalko, L. M. Nosach, S. T. Dyadyun, and N. O. Vrinchanu, in: A. V. Stefanov (editor), Preclinical Study of Medicines (Methodical Recommendations) [in Russian], Kiev (2001), p. 371.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 4, pp. 513-526, April, 2014.
Rights and permissions
About this article
Cite this article
Siry, S.A., Timoshenko, V.M., Vlasenko, Y.G. et al. Pummerer Reactions of Thiopyran Derivatives as a Method for the Preparation of Trifluoro-Methyl-Substituted Thiolanes with Antiviral Activity. Chem Heterocycl Comp 50, 467–478 (2014). https://doi.org/10.1007/s10593-014-1497-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1497-0