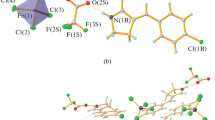
The oxidative nucleophilic alkoxylation of 1,3,7-triazapyrene under acid catalysis conditions is preceded by diprotonation of the substrate. 6,8-Dialkoxy-1-methyl-1,3,7-triazapyrenium salts were synthesized. An unusual transformation of 6,8-diethoxy- and 6,8-dipropoxy-1,3,7-triazapyrenes to 1,3-dimethyl-6,8-dioxo-1,6,7,8-tetrahydro-1,3,7-triazapyrenium salts was found.

Similar content being viewed by others
References
O. P. Demidov, I. V. Borovlev, S. V. Pisarenko, O. A. Nemykina, and N. A. Saigakova, Khim. Geterotsikl. Soedin., 791 (2010). [Chem. Heterocycl. Compd., 46, 636 (2010)].
I. V. Borovlev, O. P. Demidov, and N. A. Saigakova, Izv. Akad. Nauk, Ser. Khim., 1755 (2011). [Russ. Chem. Bull., 60, 1784 (2011)].
A. F. Pozharskii, Theoretical Fundamentals of Heterocyclic Chemistry [in Russian], Khimiya, Moscow (1985), p. 218.
I. V. Borovlev, O. P. Demidov, and N. A. Saigakova, Khim. Geterotsikl. Soedin., 662 (2013). [Chem. Heterocycl. Compd., 49, 618 (2013)].
S. V. Pisarenko, O. P. Demidov, A. V. Aksenov, and I. V. Borovlev, Khim. Geterotsikl. Soedin., 735 (2009). [Chem. Heterocycl. Compd., 45, 580 (2009)].
A. V. Aksenov, I. V. Borovlev, I. V. Aksenova, S. V. Pisarenko, and D. A. Kovalev, Tetrahedron Lett., 49, 707 (2008).
I. V. Borovlev, O. P. Demidov, S. V. Pisarenko, and O. A. Nemykina, Khim. Geterotsikl. Soedin., 597 (2010). [Chem. Heterocycl. Compd., 46, 473 (2010)].
R. Daniels, L. G. Grady, and L. Bauer, J. Am. Chem. Soc., 87, 1531 (1965).
O. P. Demidov, I. V. Borovlev, N. A. Saigakova, O. A. Nemykina, and S. V. Pisarenko, Khim. Geterotsikl. Soedin., 1639 (2012). [Chem. Heterocycl. Compd., 48, 1527 (2012)].
I. V. Borovlev, O. P. Demidov, S. V. Pisarenko, N. V. Demidova, and O. A. Nemykina, Zh. Org. Khim., 45, 1739 (2009). [Russ. J. Org. Chem., 45, 1736 (2009)].
I. V. Borovlev, O. P. Demidov, S. V. Pisarenko, O. A. Nemykina, and N. A. Saigakova, Khim. Geterotsikl. Soedin., 1146 (2012) [Chem. Heterocycl. Compd., 48, 1064 (2012)].
J. W. Bunting, Adv. Het. Chem., 25, 1 (1979).
This work was carried out with the support of the Ministry of Education and Science of the Russian Federation within the higher education program for 2013 (registry No. 3.8584.2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 12, pp. 1917-1924, December, 2013.
Rights and permissions
About this article
Cite this article
Demidov, O.P., Saigakova, N.A., Demidova, N.V. et al. Synthesis and Structure of Salts Derived from 6,8-Dialkoxy-1,3,7-Triazapyrenes. Chem Heterocycl Comp 49, 1777–1784 (2014). https://doi.org/10.1007/s10593-014-1430-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1430-6