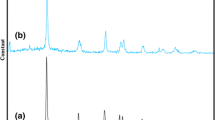
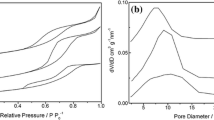
A new and environmentally benign method for the preparation of N-substituted pyrroles from one-pot condensation reaction of 2,5-dimethoxytetrahydrofuran with amines and diamines in the presence of nano sulfated titania under solvent-free conditions is presented. This new protocol features simple operation, easy availability, stability, and eco-friendliness of catalyst, as well as high to excellent yields of products. In addition, we report for the first time an alternative method for the synthesis of pyrroles from aromatic amines containing the β-lactam fragment using nano sulfated titania as catalyst.
Similar content being viewed by others
References
D. L. Boger, C. W. Boyce, M. A. Labroli, C. A. Sehon, and Q. Jin, J. Am. Chem. Soc., 121, 54 (1999).
J. T. Gupton, Top. Heterocycl. Chem., 2, 53 (2006).
H. Fan, J. Peng, M. T. Hamann, and J. F. Hu, Chem. Rev., 108, 264 (2008).
F. Aydogan, M. Basarir, C. Yolacan, and A. S. Demir, Tetrahedron, 63, 9746 (2007).
R. Paolesse, S. Nardis, F. Sagone, and R. G. Khoury, J. Org. Chem., 66, 550 (2001).
R. K. Dieter and H. Yu, Org. Lett., 2, 2283 (2000).
M. Yamagishi, K. Nishigai, T. Hata, and H. Urabe, Org. Lett., 13, 4873 (2011).
Furstner, H. Weintritt, and A. Hupperts, J. Org. Chem., 60, 6637 (1995).
Freifeld, H. Shojaei, and P. Langer, J. Org. Chem., 71, 4965 (2006).
Eftekhari-Sis, M. Zirak, and A. Akbari, Chem. Rev., 113, 2958 (2013).
E. Ghabraie, S. Balalaie, M. Bararjanian, H. R. Bijanzadeh, and F. Rominger, Tetrahedron, 67, 5415 (2011).
V. Estévez, M. Villacampa, and J. C. Menéndez, Chem. Soc. Rev., 39, 4402 (2010).
A. Jafari, H. Mahmoudi, and B. F. Mirjalili, J. Iran. Chem. Soc., 8, 851 (2011).
P. Mundy, M. G. Ellerd, and F. G. Favaloro, Jr., Name Reactions and Reagents in Organic Synthesis, 2nd ed., John Wiley & Sons, Inc. (2005), p. 476.
T. Maehara, R. Kanno, S. Yokoshima, and T. Fukuyama, Org. Lett., 14, 1946 (2012).
G. Dou, C. Shi, and D. Shi, J. Comb. Chem., 10, 810 (2008).
H. Veisi and S. Hemmati, J. Chin. Chem. Soc., 56, 240 (2009).
S. Handy and K. Lavender, Tetrahedron Lett., 54, 4377 (2013).
L. Gao, L. Bing, Z. Zhang, H. Kecheng, H. Xiaoyun, and K. Deng, J. Organomet. Chem., 735, 26 (2013).
B. S. Gourlay, P. P. Molesworth, J. H. Ryan, and J. A. Smith, Tetrahedron Lett., 47, 799 (2006).
C. Miles, S. M. Mays, B. K. Southerland, T. J. Auvil, and D. M. Ketcha, ARKIVOC, xiv, 181 (2009).
R. Bharadwaj and K. A. Scheidt, Org. Lett., 6, 2465 (2004).
C. Silveira, M. P. Fortes, and S. R. Mendes, Curr. Org. Chem., 16, 1540 (2012).
F. Aydogan and C. Yolacan, J. Chem. (2013). http://dx.doi.org/10.1155/2013/976724.
M. Abid, L. Teixeira, and B. Török, Tetrahedron Lett., 48, 4047 (2007).
M. Abid, S. M. Landge, and B. Török, Org. Prep. Proced. Int., 38, 495 (2006).
C. Rochais, V. Lisowski, P. Dallemagne, and S. Rault, Tetrahedron Lett., 45, 6353 (2004).
N. Azizi, A. Khajeh-Amiri, H. Ghafuri, M. Bolourtchian, and M. R. Saidi, Synlett, 2245 (2009).
S. Rivera, D. Bandyopadhyay, and B. K. Banik, Tetrahedron Lett., 50, 5445 (2009).
X. Zhang and J. Shi, Tetrahedron, 67, 898 (2011).
Aghapoor, L. Ebadi-Nia, F. Mohsenzadeh, M. M. Morad, Y. Balavar, and H. R. Darabi, J. Organomet. Chem., 708, 25 (2012).
H. Veisi, Tetrahedron Lett., 51, 2109 (2010).
J. Chen, H. Wu, Z. Zheng, C. Jin, X. Zhang, and W. Su, Tetrahedron Lett., 47, 5383 (2006).
D. Bandyopadhyay, S. Mukherjee, J. C. Granados, J. D. Short, and B. K. Banik, Eur. J. Med. Chem., 50, 209 (2012).
A. Wilson, G. Filzen and G. S. Welmaker, Tetrahedron Lett., 50, 4807 (2009).
V. Polshettiwar and R. S. Varma, Tetrahedron, 66, 1091 (2010).
Y. Fang, D. Leysen, and H. C. J. Ottenheijm, Synth. Commun., 25, 1857 (1995).
Hosseini-Sarvari, E. Sodagar, and M. M. Doroodmand, J. Org. Chem., 76, 2853 (2011).
Hosseini-Sarvari and E. Safari, J. Sulfur Chem., 32, 463 (2011).
M. Hosseini-Sarvari, E. Safari, A. Jarrahpour, and R. Heiran, C. R. Chim., 15, 980 (2012).
M. Zarei, A. Jarrahpour, E. Ebrahimi, M. Aye, and S. A. Torabi Badrabady, Tetrahedron, 68, 5505 (2012).
L. K. Noda, R. M. de Almeida, L. F. D Probst, and N. S. Goncalves, J. Mol. Catal. A: Chem., 225, 39 (2005).
X. Song and A. Sayari, Catal. Rev. Sci. Eng., 38, 329 (1996).
H.-C. Ma and X.-Z. Jiang, J. Org. Chem., 72, 8943 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, No. 12, pp. 1870-1877, December, 2013.
Rights and permissions
About this article
Cite this article
Hosseini-Sarvari, M., Najafvand-Derikvandi, S., Jarrahpour, A. et al. Nano Sulfated Titania as a Heterogeneous Solid Acid Catalyst for the Synthesis of Pyrroles by Clauson–Kaas Condensation under Solvent-free Conditions. Chem Heterocycl Comp 49, 1732–1739 (2014). https://doi.org/10.1007/s10593-014-1425-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1425-3