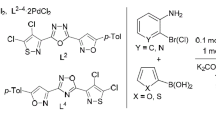
We used 5-arylisoxazole-3-carbonitriles and 4-R-5-chloroisothiazole-3-carbonitriles to prepare the corresponding (5-arylisoxazol-3-yl)- and (4-R-5-chloroisothiazol-3-yl)-1,2,4-triazoles, -tetrazoles, and -1,3,4-oxadiazoles. The obtained bis-heterocycles along with some of the intermediate monocyclic azoles formed palladium complexes that were highly active Suzuki reaction catalysts in aqueous and aqueous-alcohol media.
Similar content being viewed by others
References
J. J. Talley, D. L. Brown, J. S. Carter, M. J. Graneto, C. M. Koboldt, J. L. Masferrer, W. E. Perkins, R. S. Rogers, A. F. Shaffer, Y. Y. Zhang, B. S. Zweifel, and K. Seibert, J. Med. Chem., 43, 775 (2000).
T. S. Gardner, E. Wenis, and J. Lee, J. Med. Chem., 2, 133 (1960).
P. Pinto and M. Dougados, Acta Reumatológica Portuguesa, 31, 215 (2006).
M. Shailaja, A. Manjula, and B. Vittal Rao, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 50B, 214 (2011).
J. S. Beebe, J. P. Jani, E. Knauth, P. Goodwin, C. Higdon, A. M. Rossi, E. Emerson, M. Finkelstein, E. Floyd, S. Harriman, J. Atherton, S. Hillerman, C. Soderstrom, K. Kou, T. Gant, M. C. Noe, B. Foster, F. Rastinejad, M. A. Marx, T. Schaeffer, P. M. Whalen, and W. G. Roberts, Cancer Res., 63, 7301 (2003).
V. A. Kulchitsky, V. I. Potkin, Yu. S. Zubenko, A. N. Chernov, M. V. Talabaev, Yu. E. Demidchik, S. K. Petkevich, V. V. Kazbanov, T. A. Gurinovich, M. O. Roeva, D. G. Grigoriev, A. V. Kletskov, and V. N. Kalunov, Med. Chem., 8, 22 (2012).
V. Potkin, Y. Zubenko, A. Bykhovetz, R. Zolotar, and V. Goncharuk, Nat. Prod. Commun., 4, 1205, (2009).
V. I. Potkin, N. A. Bumagin, V. M. Zelenkovsky, S. K. Petkevich, Yu. S. Zubenko, M. V. Livantsov, and D. S. Belov, Dokl. Nat. Acad. Nauk Belarusi, 5, 52 (2011).
V. I. Potkin, N. A. Bumagin, S. K. Petkevich, A. S. Lyakhov, D. A. Rudakov, M. V. Livantsov, and N. E. Golantsov, Synthesis, 44, 151 (2012).
S. V. Voitekhovich, Т. V. Serebryanskaya, A. S. Lyakhov, P. N. Gaponik, and O. A. Ivashkevich, Polyhedron, 28, 3614 (2009).
P. N. Gaponik, S. V. Voytekhovich, and O. A. Ivashkevich, Usp. Khim., 75, 569 (2006).
S. Komeda, M. Lutz, A. L. Spek, Y. Yamanaka, T. Sato, M. Chikuma, and J. Reedijk, J. Am. Chem. Soc., 124, 4738 (2002).
V. Padmavathi, G. Sudhakar Reddy, A. Padmaja, P. Kondaiah, and Ali-Shazia, Eur. J. Med. Chem., 44, 2106 (2009).
N. B. Patel, A. C. Purohit, D. P. Rajani, R. Moo-Puc, and G. Rivera, Eur. J. Med. Chem., 62, 677 (2013).
V. I. Potkin, R. A. Gadzhili, E. A. Dikusar, S. K. Petkevich, N. A. Zhukovskaya, A. G. Aliev, and Sh. F. Nagieva, Zh. Org. Khim, 48, 132 (2012).
V. I. Potkin, Yu. S. Zubenko, N. I. Nechay, A. I. Bikhovets, and P. V. Kurman, Zh. Org. Khim., 44, 1048 (2008).
E. N. Zil’berman, Nitrile reactions [in Russian], Khimiya, Moscow (1972), p. 169.
G. Rousselet, P. Capdevielle, and M. Maumy, Tetrahedron Lett., 34, 6395 (1993).
D. G. Neilson, R. Roger, J. W. M. Heatlie, and L. R. Newlands, Chem. Rev., 70, 151 (1970).
V. Ya. Grinshtein, A. A. Strazdin’, and A. K. Grinvalde, Khim. Geterotsikl. Soedin., 248 (1970). [Chem. Heterocycl. Compd., 6, 231 (1970).]
V. V. Vishnyakov, P. P. Purygin, I. A. Potapova, and S. V. Pan’kov, Vest. Samara State Univ. Nat. Sci. Ser., 34, 132 (2004).
N. A. Bumagin, I. O. Kalinovsky, A. B. Ponomaryov, and I. P. Beletskaya, Dokl. Akad. Nauk SSSR, 265, 1138 (1982).
N. A. Bumagin, I. G. Bumagina, and I. P. Beletskaya, Dokl. Akad. Nauk SSSR, 274, 818 (1984).
N. A. Bumagin, A. B. Ponomaryov, and I. P. Beletskaya, J. Organomet. Chem., 291, 129 (1985).
N. A. Bumagin, Y. V. Gulevich, and I. P. Beletskaya, J. Organomet. Chem., 285, 415 (1985).
N. A. Bumagin, D. A. Tsarev, Tetrahedron Lett., 39, 8155 (1998).
European Medicines Agency, Guideline on the Specification Limits for Residues of Metals Catalysts, January 2007, p. 6: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003587.pdf
Z. Zhou, Q. Hu, Z. Du, J. Xue, S. Zhang, and Y. Xie, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 42, 940 (2012).
V. Pomel, J. Klicic, D. Covini, D. D. Church, J. P. Shaw, K. Roulin, F. Burgat-Charvillon, D. Valognes, M. Camps, C. Chabert, C. Gillieron, B. Françon, D. Perrin, D. Leroy, D. Gretener, A. Nichols, P. A. Vitte, S. Carboni, C. Rommel, M. K. Schwarz, and T. Rückle, J. Med. Chem., 49, 3857 (2006).
P. Villain-Guillot, M. Gualtieri, L. Bastide, F. Roquet, J. Martinez, M. Amblard, M. Pugniere, and J.-P. Leonetti, J. Med. Chem., 50, 4195 (2007).
A. R. Katritzky, S. R. Tala, H. Lu, A. V. Vakulenko, Q.-Y. Chen, J. Sivapackiam, K. Pandya, S. Jiang, and A. K. Debnath, J. Med. Chem., 52, 7631 (2009).
B. E. Sleebs, W. J. A. Kersten, S. Kulasegaram, G. Nikolakopoulos, E. Hatzis, R. M. Moss, J. P. Parisot, H. Yang, P. E. Czabotar, W. Douglas Fairlie, E. F. Lee, J. M. Adams, L. Chen, M. F. van Delft, K. N. Lowes, A. Wei, D. C. S. Huang, P. M. Colman, I. P. Street, J. B. Baell, K. Watson, and G. Lessene, J. Med. Chem., 56, 5514 (2013).
J. Hannah, W. V. Ruyle, H. Jones, A. R. Matzuk, K. W. Kelly, B. E. Witzel, W. J. Holtz, R. A. Houser, and T. Y. Shen, J. Med. Chem., 21, 1093 (1978).
R. B. De Vasher, L. R. Moore, and K. H. Shaughnessy, J. Org. Chem., 69, 7919 (2004).
Al Steyermark, Quantitative Organic Microanalysis, Academic Press, New York, London (1961).
W. G. Dauben and M. Tanabe, J. Am. Chem. Soc., 75, 4969 (1953).
D. N. Korolev and N. A. Bumagin, Tetrahedron Lett., 46, 5751 (2005).
This work was carried out with financial support from the Russian Foundation for Basic Research (grants 12-08-90025-Bel_а, 11-08-00353-а) and the Belarusian Republican Foundation for Fundamental Research (grant H12R-024).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 10, pp. 1633-1649, October, 2013.
Rights and permissions
About this article
Cite this article
Bumagin, N.A., Petkevich, S.K., Kletskov, A.V. et al. Isoxazol-3-yl(Isothiazol-3-yl)-1,2,4-Triazoles, Tetrazoles, and -1,3,4-Oxadiazoles: Synthesis, Palladium Complexes, and Catalytic Applications. Chem Heterocycl Comp 49, 1515–1529 (2014). https://doi.org/10.1007/s10593-014-1403-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1403-9