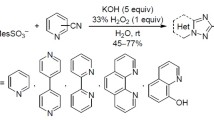
The reaction of 3-alkynylquinoxaline-2-carbonitriles with sodium azide in DMF at 60 °C leads to the formation of 4,5-disubstituted 2H-1,2,3-triazoles in 38-82% yields. The analogous reaction in toluene in the presence of AlCl3 takes place as a tandem process involving 1,3-dipolar cycloaddition of the azide ion to the nitrile group followed by 6-endo-digonal cyclization with the formation of 5-aryl-tetrazolo[1′,5′:1,3]pyrido[3,4-b]quinoxalines.


Similar content being viewed by others
References
E. A. Glazer and L. R. Chappel, J. Med. Chem., 25, 766 (1982).
E. A. Glazer and J. E. Presslitz, J. Med. Chem., 25, 868 (1982).
J. Harmenberg, A. Åkesson-Johansson, A. Gräslund, T. Malmfors, J. Bergman, B. Wahren, S. Åkerfeldt, L. Lundblad, and S. Cox, Antiviral Res., 15, 193 (1991).
L. E. Seitz, W. J. Suling, and R. C. Reynolds, J. Med. Chem., 45, 5604 (2002).
A. Burguete, E. Pontiki, D. Hadjipavlou-Litina, R. Villar, E. Vicente, B. Solano, S. Ancizu, S. Pérez-Silanes, I. Aldana, and A. Monge, Bioorg. Med. Chem. Lett., 17, 6439 (2007).
C. Barea, A. Pabón, S. Pérez-Silanes, S. Galiano, G. Gonzalez, A. Monge, E. Deharo, and I. Aldana, Molecules, 18, 4718 (2013).
K. Watanabe, H. Oguri, and H. Oikawa, Curr. Opin. Chem. Biol., 13, 189 (2009).
P. Thirumurugan, D. Muralidharan, and P. T. Perumal, Dyes Pigm., 81, 245 (2009).
K. R. J. Thomas, J. T. Lin, Y.-T. Tao, and C. H. Chuen, J. Mater. Chem., 12, 3516 (2002).
M. Ananth Reddy, A. Thomas, G. Mallesham, and B. Sridhar, V. Jayathirtha Rao, K. Bhanuprakash, Tetrahedron Lett., 52, 6942 (2011).
P. Wang, Z. Xie, Z. Hong, J. Tang, O. Wong, C.-S. Lee, N. Wong, and S. Lee, J. Mater. Chem., 13, 1894 (2003).
R. Chinchilla and C. Nájera, Chem. Rev., 107, 874 (2007).
N. Sato, in: A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven, and R. J. K. Taylor (editors), Comprehensive Heterocyclic Chemistry III, Vol. 8, Elsevier, Amsterdam (2008), p. 273.
A. Keivanloo, M. Bakherad, and A. Rahimi, Synthesis, 1599 (2010).
M. Bakherad, A. Keivanloo, and S. Samangooei, Tetrahedron Lett., 53, 1447 (2012).
A. V. Gulevskaya, H. T. L. Nguen, A. S. Tyaglivy, and A. F. Pozharskii, Tetrahedron, 68, 488 (2012).
P. Roy and B. K. Ghorai, Tetrahedron Lett., 53, 235 (2012).
P. Roy and K. Ghorai, Beilstein J. Org. Chem., 6, No. 52, DOI: 10.3762/bjoc.6.52 (2010).
A. V. Gulevskaya, R. Yu. Lazarevich, and A. F. Pozharskii, Tetrahedron, 69, 910 (2013).
A. V. Gulevskaya, S. V. Dang, A. S. Tyaglivy, A. F. Pozharskii, O. N. Kazheva, A. N. Chekhlov, and O. A. Dyachenko, Tetrahedron, 66, 146 (2010).
M. Meldal and C. W. Tornøe, Chem. Rev., 108, 2952 (2008).
P. D. Jarowski, Y.-L. Wu, W. B. Schweizer, and F. Diederich, Org. Lett., 10, 3347 (2008).
W. Zeng, A. Degterev, E. Hsu, J. Yuan, and C. Yuan, Bioorg. Med. Chem. Lett., 18, 4932 (2008).
C.-W. Tsai, S.-C. Yang, Y.-M. Liu, and M.-J. Wu, Tetrahedron, 65, 8367 (2009).
A. S. Tyaglivy, A. V. Gulevskaya, A. F. Pozharskii, and O. I. Askalepova, Tetrahedron, 69, DOI: 10.1016/j.tet.2013.09.005 (2013).
V. A. Ostrovskii, G. I. Koldobskii, and R. E. Trifonov, in: A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven, and R. J. K. Taylor (editors), Comprehensive Heterocyclic Chemistry III, Vol. 6, Elsevier, Amsterdam (2008), p. 257.
Y. Zhao, N. E. Schultz, and D. G. Truhlar, J. Chem. Theory Comput., 2, 364 (2006).
V. A. Rassolov, M. A. Ratner, J. A. Pople, P. C. Redfern, and L. A. Curtiss, J. Comput. Chem., 22, 976 (2001).
T. Clark, J. Chandrasekhar, G. W. Spitznagel, and P. V. R. Schleyer, J. Comput. Chem., 4, 294 (1983).
M. J. Frisch, J. A. Pople, and J. S. Binkley, J. Chem. Phys., 80, 3265 (1984).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian 03, Revision E.01, Gaussian Inc., Wallingford (2004).
A. E. Reed, L. A. Curtiss, and F. Weinhold, Chem. Rev., 88, 899 (1988).
R. M. Minyaev, Usp. Khim., 63, 939 (1994). [Russ. Chem. Rev., 63, 883 (1994).]
The work was carried out with financial support from the Russian Foundation for Basic Research (grant No. 11-03-00079).
The authors thank Z. A. Starikova* (Institute of Organoelement Compounds, Russian Academy of Sciences, Moscow) for carrying out the X-ray structural investigation.
*Deceased
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 9, pp. 1346-1355, September, 2013.
Rights and permissions
About this article
Cite this article
Tyaglivy, A.S., Steglenko, D.V. & Gulevskaya, A.V. Reaction of 3-Alkynylquinoxaline-2-carbonitriles with Sodium Azide: an Experimental and Theoretical Study. Chem Heterocycl Comp 49, 1255–1263 (2013). https://doi.org/10.1007/s10593-013-1373-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-013-1373-3