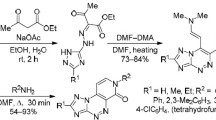
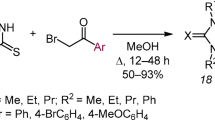
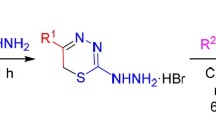
Previously unknown (Z)-6-aryl(hetaryl)methylidene derivatives of imidazo[4,5-e][1,3]thiazolo[3,2-b]-[1,2,4]triazine-2,7-diones were synthesized by aldol-crotonic condensation of imidazo[4,5-e]-[1,3]thiazolo[3,2-b][1,2,4]triazine-2,7-diones with aromatic or heteroaromatic aldehydes, or by three-component condensation of imidazo[4,5-e][1,2,4]triazine-3-thione, bromoacetic acid, and aldehyde.

Similar content being viewed by others
References
M. N. Nasr, M. M. Gineinah, and E. R. El-Bendary, Arch. Pharm., 336, 560 (2003).
S. N. Sriharsha, S. Satish, S. Shashikanth, and K. A. Raveesha, Bioorg. Med. Chem., 14, 7476 (2006).
I. R. Siddiqui, P.K. Singh, J. Singh, and J. Singh, J. Agric. Food Chem., 51, 7062 (2003).
V. Gududuru, E. Hurh, J. T. Dalton, and D. D. Miller, Bioorg. Med. Chem. Lett., 14, 5289 (2004).
R. Ottanà, S. Carotti, R. Maccari, I. Landani, G. Chiricosta, B. Caciagli. M. G. Vigorita, and E. Mini, Bioorg. Med. Chem. Lett., 15, 3930 (2005).
J. Balzarini, B. Orzeszko-Krzesińska, J. K. Maurin, and A. Orzeszko, Eur. J. Med. Chem., 44, 303 (2009).
J. P. Devlin and K. D. Hargrave, Tetrahedron, 45, 4327 (1989).
W. K. P. Amery and J. P. M. Bruynseels, Int. J. Immunopharmacol., 14, 481 (1992).
D. I. Trepanier and P. E. Krieger, US Pat. Appl. 3641019.
R. M. Abdel-Rahman, M. Seada, M. Fawzy, and I. El-Baz, Boll. Chim. Farm.,133, 381 (1994).
R. M. Abdel-Rahman, M. Seada, M. Fawzy, and I. El-Baz, Pharmazie, 49, 729 (1994).
H. Zhou, S. Wu, S. Zhai, A. Liu, Y. Sun, R. Li, Y. Zhang, S. Ekins, P. W. Swaan, B. Fang, B. Zhang, and B. Yan, J. Med. Chem., 51, 1242 (2008).
M. E. Jung, J.-M. Ku, L. Du, H. Hu, and R. A. Gatti, Bioorg. Med. Chem. Lett., 21, 5842 (2011).
M. Mushtaque, F. Avecilla, and A. Azam, Eur. J. Med. Chem., 55, 439 (2012).
S. V. Vasilevskii, P. A. Belyakov, G. A. Gazieva, Yu. V. Nelyubina, N. G. Kolotyrkina, and A. N. Kravchenko, Mendeleev. Commun., 20, 47 (2010).
G. A. Gazieva, P. A. Poluboyarov, Yu. V. Nelyubina, M. I. Struchkova, and A. N. Kravchenko, Khim. Geterotsiklich. Soed., 1483 (2012). [Chem. Heterocycl. Compd., 48, 1382 (2012)].
R. Ottanà, R. Maccari, M. L. Barreca, G. Bruno, A. Rotondo, A. Rossi, G. Chiricosta, R. Di Paola, L. Sautebin, S. Cuzzocrea, M. G. Vigorita, and E. Mini, Bioorg. Med. Chem., 13, 4243 (2005).
O. Bozdağ-Dundar, Ö. Özgen, A. Menteşe, N. Altanlar, O. Atli, E. Kendi, and R. Ertan, Bioorg. Med. Chem., 15, 6012 (2007).
R. Lesyk, O. Vladzimirska, S. Holota, L. Zaprutko, and A. Gzella, Eur. J. Med. Chem., 42, 641 (2007).
S. V. Vasilevskii, Yu. V. Nelyubina, N. G. Kolotyrkina, P. A. Belyakov, L. B. Kulikova, and A. N. Kravchenko, Mendeleev. Commun., 20, 288 (2010).
S. A. Sigachev, A. N. Kravchenko, K. A. Lyssenko, P. A. Belyakov, O. V. Lebedev, and N. N. Makhova, Mendeleev. Commun., 13, 190 (2003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 7, 1176-1180, July, 2013.
Rights and permissions
About this article
Cite this article
Gazieva, G.A., Serkov, S.A., Sigai, N.V. et al. Synthesis of New Imidazo[4,5-e][1,3]thiazolo-[3,2-b][1,2,4]triazine Derivatives. Chem Heterocycl Comp 49, 1097–1101 (2013). https://doi.org/10.1007/s10593-013-1349-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-013-1349-3