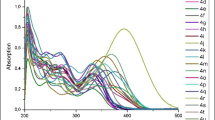
Prop-2-enylidene indolones substituted with L-amino acid esters are obtained in good to excellent yields in a consecutive three-component cyclocarbopalladation, Sonogashira coupling, and Michael addition sequence. While primary L-amino acid esters furnish a mixture of E,E- and E,Z-configurated diastereomers, L-proline methyl ester selectively and exclusively furnishes the E,E-isomer. As already discovered for other 3-aminoprop-2-enylidene indolones, the drop-casted films of all representatives display pronounced aggregation-induced orange-red fluorescence with large Stokes shifts, while all chromophores are nonemissive in solution.



Similar content being viewed by others
References
T. J. J. Müller, and U. H. F. Bunz (editors), Functional Organic Materials. Syntheses, Strategies, and Applications, Wiley-VCH, Weinheim (2007).
K. Müllen, and G. Wegner (editors), Electronic Materials: the Oligomer Approach, Wiley-VCH, Weinheim (1998).
K. Müllen and U. Scherf (editors), Organic Light-Emitting Diodes – Synthesis, Properties, and Applications, Wiley-VCH, Weinheim (2006).
E. Kim and S. B. Park, Chem.-Asian J., 4, 1646 (2009).
C. W. Cairo, J. A. Key, and C. M. Sadek, Curr. Opin. Chem. Biol., 14, 57 (2010).
H.-A. Wagenknecht, Ann. NY Acad. Sci., 1130, 122 (2008).
J. Zhu and H. Bienaymé (editors), Multicomponent Reactions, Wiley-VCH, Weinheim (2005).
J. D. Sunderhaus and S. F. Martin, Chem.–Eur. J., 15, 1300 (2009).
N. Isambert and R. Lavilla, Chem.-Eur. J., 14, 8444 (2008).
A. Dömling, Chem. Rev., 106, 17 (2006).
R. V. A. Orru and M. de Greef, Synthesis, 1471 (2003).
H. Bienaymé, C. Hulme, G. Oddon, and P. Schmitt, Chem.-Eur. J., 6, 3321 (2000).
A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 39, 3168 (2000).
I. Ugi, A. Dömling, and B. Werner, J. Heterocycl. Chem., 37, 647 (2000).
L. Weber, K. Illgen, and M. Almstetter, Synlett, 366 (1999).
R. W. Armstrong, A. P. Combs, P. A. Tempest, S. D. Brown, and T. A. Keating, Acc. Chem. Res., 29, 123 (1996).
I. Ugi, A. Dömling, and W. Hörl, Endeavour, 18, 115 (1994).
G. H. Posner, Chem. Rev., 86, 831 (1986).
L. F. Tietze, G. Brasche, and K. M. Gericke (editors), Domino Reactions in Organic Synthesis, Wiley-VCH, Weinheim (2006).
L. F. Tietze, Chem. Rev., 96, 115 (1996).
L. F. Tietze and U. Beifuss, Angew. Chem., Int. Ed. Engl., 32, 131 (1993).
L. F. Tietze, J. Heterocycl. Chem., 27, 47 (1990).
S. L. Schreiber and M. D. Burke, Angew. Chem., Int. Ed., 43, 46 (2004).
M. D. Burke, E. M. Berger, and S. L. Schreiber, Science, 302, 613 (2003).
D. R. Spring, Org. Biomol. Chem., 1, 3867 (2003).
P. Arya, D. T. H. Chou, and M. G. Baek, Angew. Chem., Int. Ed., 40, 339 (2001).
B. Cox, J. C. Denyer, A. Binnie, M. C. Donnelly, B. Evans, D. V. S. Green, J. A. Lewis, T. H. Mander, A. T. Merritt, M. J. Valler, and S. P. Watson, Prog. Med. Chem., 37, 83 (2000).
S. L. Schreiber, Science, 287, 1964 (2000).
T. J. J. Müller and D. M. D'Souza, Pure Appl. Chem., 80, 609 (2008).
T. J. J. Müller, in: T. J. J. Müller and U. H. F. Bunz (editors), Functional Organic Materials. Syntheses, Strategies, and Applications, Wiley-VCH, Weinheim (2007), p. 179.
C. A. Briehn and P. Bäuerle, Chem. Commun., 1015 (2002).
D. M. D'Souza and T. J. J. Müller, Chem. Soc. Rev., 36, 1095 (2007).
G. Balme, E. Bossharth, and N. Monteiro, Eur. J. Org. Chem., 4101 (2003).
G. Battistuzzi, S. Cacchi, and G. Fabrizi, Eur. J. Org. Chem., 2671 (2002).
T. J. J. Müller, Synthesis, 44, 159 (2012).
T. J. J. Müller, Top. Heterocycl. Chem., 25, 25 (2010).
B. Willy and T. J. J. Müller, Curr. Org. Chem., 13 , 1777 (2009).
B. Willy and T. J. J. Müller, ARKIVOC, i, 195 (2008).
T. J. J. Müller, Targets Heterocycl. Syst., 10, 54 (2006).
A. S. Karpov, F. Rominger, and T. J. J. Müller, J. Org. Chem., 68, 1503 (2003).
T. J. J. Müller, J. P. Robert, E. Schmälzlin, C. Bräuchle, and K. Meerholz, Org. Lett., 2, 2419 (2000).
M. Teiber and T. J. Müller, Chem. Commun. (Cambridge, U. K.), 48, 2080 (2012).
O. Grotkopp, A. Ahmad, W. Frank, and T. J. J. Müller, Org. Biomol. Chem., 9, 8130 (2011).
B. Willy and T. J. J. Müller, Org. Lett., 13, 2082 (2011).
S. Rotzoll, B. Willy, J. Schönhaber, F. Rominger, and T J. J. Müller, Eur. J. Org. Chem., 3516 (2010).
B. Willy and T. J. J. Müller, Eur. J. Org. Chem., 4157 (2008).
B. Willy, T. Dallos, F. Rominger, J. Schönhaber, and T. J. J. Müller, Eur. J. Org. Chem., 4796 (2008).
O. G. Schramm, née Dediu, T. Oeser, and T. J. J. Müller, J. Org. Chem., 71, 3494 (2006).
A. V. Rotaru, I. D. Druta, T. Oeser, and T. J. J. Müller, Helv. Chim. Acta., 88, 1798 (2005).
R. U. Braun and T. J. J. Müller, Synthesis, 2391 (2004).
J. Schönhaber and T. J. J. Müller, Org. Biomol. Chem., 9, 6196 (2011).
D. M. D'Souza, A. Kiel, D.-P. Herten, and T. J. J. Müller, Chem.-Eur. J., 14, 529 (2008).
D. M. D'Souza, F. Rominger, and T. J. J. Müller, Angew. Chem., Int. Ed., 44, 153 (2005).
J. Schönhaber, W. Frank, and T. J. J. Müller, Org. Lett., 12, 4122 (2010).
D. M. D'Souza, C. Muschelknautz, F. Rominger, and T. J. J. Müller, Org. Lett., 12, 3364 (2010).
C. Muschelknautz, W. Frank, and T. J. J. Müller, Org. Lett., 13, 2556 (2011).
G. Bach and S. Daehne, in: M. Sainsbury (editor), Rodd's Chemistry of Carbon Compounds, Vol. IVB, Amsterdam, Elsevier (1997), p. 383.
F. Würthner and K. Meerholz, Chem.-Eur. J., 16, 9366 (2010).
S. Dähne, Chimia, 45, 288 (1991).
N. C. Craig, P. Groner, and D. C. McKean, J. Phys. Chem. A, 110 , 7461 (2006).
S. R. Marder, J. W. Perry, B. G. Tiemann, C. B. Gorman, S. Gilmour, S. L. Biddle, and G. Bourhill, J. Am. Chem. Soc., 115, 2524 (1993).
SPARTAN '08 V 1.2.0, Wavefunction Inc., Irvine (2008).
A. Mishra, R. K. Behera, P. K. Behera, B. K. Mishra, and G. B. Behera, Chem. Rev., 100 , 1973 (2000).
A. V. Kulinich and A. A. Ishenko, Russ. Chem. Rev., 78, 141 (2009).
W. J. Harrison, D. L. Mateer, and G. J. T. Tiddy, J. Phys. Chem., 100, 2310 (1996).
Y. Hong, J. W. Y. Lam, and B. Z. Tang, Chem. Soc. Rev., 40, 5361 (2011).
Y. Hong, J. W. Y. Lam, and B. Z. Tang, Chem. Commun., 4332 (2009).
A. S. Davydov, Theory of Molecular Excitons, Plenum Press, New York, London (1971).
H. G. O. Becker, R. Beckert, G. Domschke, E. Fanghänel, W. D. Habicher, P. Metz, D. Pavel, and K. Schwetlick (editors), Organikum, 22nd ed., Wiley-VCH, Weinheim, New York, Chichester, Brisbane, Singapore, Toronto (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, No. 6, pp. 922–934, June, 2013.
Rights and permissions
About this article
Cite this article
Muschelknautz, C., Mayer, B., Rominger, F. et al. Consecutive three-component synthesis of film luminescent indolone merocyanines with L-amino acid ester donors. Chem Heterocycl Comp 49, 860–871 (2013). https://doi.org/10.1007/s10593-013-1320-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-013-1320-3