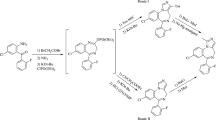
It was shown by 1H and 13C NMR spectroscopy that the 2-aryl-6-oxohexahydropyrimidine-4-carboxylic acid sodium salts existed in D2O solution as a tautomeric mixture of cyclic and linear forms. The cyclic form consisted of two (2RS,4S)-stereoisomers, differing in the aryl substituent configuration at the pyrimidine ring C-2 atom.
Similar content being viewed by others
References
K. N. Zelenin, V. V. Alekseyev, I. V. Ukraintsev, and I. V. Tselinsky, Org. Prep. Proc. Int., 30, 53 (1998).
A. D. Shutalev, M. T. Pagaev, and L. A. Ignatova, Khim. Geterotsikl. Soedin., 1093 (1994). [Chem. Heterocycl. Compd., 30, 943 (1994).]
L. Lázár and F. Fülöp, Eur. J. Org. Chem., 3025 (2003).
K. N. Zelenin, V. V. Alekseyev, K. Pikhlaia, and V. V. Ovcharenko, Izv. Akad. Nauk, Ser. Khim., 197 (2002).
R. E. Valters, F. Fűlöp, and D. Korbonits, Adv. Heterocycl. Chem., 64, 251 (1995).
R. E. Valter, Ring-chain Isomerism in Organic Chemistry [in Russian], Zinatne, Riga (1978), 238 pp.
J. P. Konopelski, L. K. Filonova, and M. M. Olmstead, J. Am. Chem. Soc., 119, 4305 (1997).
E. Juaristi, D. Quintana, M. Balderas, and E. Garcia-Pérez, Tetrahedron Asymmetry, 7, 2233 (1996).
F. J. Lakner and G. R. Negrete, Synlett, 643 (2002).
A. M. Mfuh, M. P. D. Mahindaratne, M. V. Quintero, F. J. Lakner, A. Bao, B. A. Goins, W. T. Phillips, and G. R. Negrete, Langmuir, 27, 4447 (2011).
M. Nahrwold, A. Stončius, A. Penner, B. Neumann, H. G. Stammler, and N. Sewald, Beilstein J. Org. Chem., 5, 43 (2009).
K. S. Chu, G. R. Negrete, J. P. Konopelski, F. J. Lakner, N.-T. Woo, and M. M. Olmstead, J. Am. Chem. Soc., 114, 1800 (1992).
S. K. Kundu, M. P. D. Mahindaratne, B. Quinones, G. R. Negrete, and E. R. T. Tiekink, Acta Crystallogr., Sect. E: Struct. Rep. Online, E66, o3 (2010).
Yu. A. Zhdanov and V. I. Minkin, Correlation Analysis in Organic Chemistry [in Russian], Izd. Rost. Univ., Rostov-on-the-Don (1966), 470 pp. 15.
A. Yu. Ershov, T. V. Susarova, B. V. Chernitsa, and V. V. Shamanin, RF Pat. 2455287.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 4, pp. 640–645, April, 2013.
Rights and permissions
About this article
Cite this article
Ershov, A.Y., Nasledov, D.G., Nasonova, K.V. et al. Ring-chain tautomerism of 2-aryl-6-oxohexahydropyrimidine-4-carboxylic acid sodium salts. Chem Heterocycl Comp 49, 598–603 (2013). https://doi.org/10.1007/s10593-013-1287-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-013-1287-0