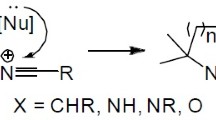
Published examples of the polynuclear fused heterocyclic systems production by cycloaddition reactions involving aryne intermediates generated in situ are reviewed.
Similar content being viewed by others
Notes
D. S. Kopchuk, G. V. Zyryanov, I. S. Kovalev, I. N. Egorov, V. L. Rusinov, O. N. Chupakhin, Khim. Geterotsikl. Soedin., in press.
References
Y. Kashiwada, A. Aoshima, Y. Ikeshiro, Y.-P. Chen, H. Furukawa, M. Itoigawa, T. Fujioka, K. Mihashi, L. M. Cosentino, S. L. Morris-Natschke, and K.-H. Lee, Bioorg. Med. Chem., 13, 443 (2005).
L. L. Bambas (editor), Chemistry of Heterocyclic Compounds: Five-Member Heterocyclic Compounds with Nitrogen and Sulfur or Nitrogen, Sulfur and Oxygen (Except Thiazole), Vol. 4, Published Online 02.01.2008, Print ISBN 9780470375877, Online ISBN 9780470186534. DOI 10.1002/9780470186534.
P. Rigo, W. Baratta, K. Siega, G. A. Chelucci, M. Ballico, and S. Magnolia, US Pat. Appl. 20100152448.
T. Gunnlaugsson, M. Glynn, G. M. Tocci (nee Hussey), P. E. Kruger, and F. M. Pfeffer, Coord. Chem. Rev., 250, 3094 (2006).
M. V. Nandakumar, S. Ghosh, and C. Schneider, Eur. J. Org. Chem., 6393 (2009).
A. Tsuboyama, H. Iwawaki, M. Furugori, T. Mukaide, J. Kamatani, S. Igawa, T. Moriyama, S. Miura, T. Takiguchi, S. Okada, M. Hoshino, and K. Ueno, J. Am. Chem. Soc., 125, 12971 (2003).
Z. H. Skraup, Monatsh. Chem., 1, 139 (1881).
F. Kröhnke, Synthesis, 1 (1976).
G. R. Pabst, O. C. Pfüller, and J. Sauer, Tetrahedron, 55, 8045 (1999).
A. Rykowski, D. Branowska, and J. Kielak, Tetrahedron Lett., 41, 3657 (2000).
V. N. Kozhevnikov, O. V. Shabunina, D. S. Kopchuk, M. M. Ustinova, B. König, and D. N. Kozhevnikov, Tetrahedron, 64, 8963 (2008).
Z. Liu, Novel Aryne Chemistry in Organic Synthesis, Iowa State University (2006), 186 p.
S. A. Worlikar and R. C. Larock, Curr. Org. Chem., 15, 3214 (2011).
H. Pellissier and M. Santelli, Tetrahedron, 59, 701 (2003).
H. H. Wenk, M. Winkler, and W. Sander, Angew. Chem., Int. Ed., 42, 502 (2003).
K. M. Allan, C. D. Gilmore, and B. M. Stoltz, Angew. Chem., Int. Ed., 50, 4488 (2011).
A. E. Goetz, S. M. Bronner, J. D. Cisneros, J. M. Melamed, R. S. Paton, K. N. Houk, and N.K. Garg, Angew. Chem. Int. Ed., 51, 2758 (2012).
R. Sanz, Org. Prep. Proced. Int., 40, 219 (2008).
R. Webster and M. Lautens, Org. Lett., 11, 4688 (2009).
K. Shahlai, S. O. Acquaah, and H. Hart, Org. Synth., 75, 201 (1998).
F. M. Raymo, M. F. Parisi, and F. H. Kohnke, Tetrahedron Lett., 34, 5331 (1993).
G. Wittig and G. Reichel, Chem. Ber., 96,2851 (1963).
M. Lautens, A. Fagnou, and V. Zuniс, Org. Lett., 4, 3465 (2002).
P. S. Anderson, M. E. Christy, G. F. Lundell, and G. S. Ponticello, Tetrahedron Lett., 16, 2553 (1975).
R. D. Giacometti and Y. K. Ramtohul, Synlett, 2010 (2009).
T. Thiemann, H. Fujii, D. Ohira, K. Arima, Y. Li, and S. Mataka, New J. Chem., 27, 1377 (2003).
P. M. Jackson and C. J. Moody, J. Chem. Soc., Perkin Trans. 1, 681 (1990).
P. M. Tadross, C. D. Gilmore, P. Bugga, S. C. Virgil, and B. M. Stoltz, Org. Lett., 12, 1224 (2010).
C. Gonzalez, E. Guitian, and L. Castedo, Tetrahedron Lett., 37, 405 (1996).
A. A. Aly, N. K. Mohamed, A. A. Hassan, and A.-F. E. Mourad, Tetrahedron, 55, 1111 (1999).
H. Hussain, E. Kianmehr, and T. Durst, Tetrahedron Lett., 42, 2245 (2001).
T. Kitamura, N. Fukatsu, and Y. Fujiwara, J. Org. Chem., 63, 8579 (1998).
R. C. Cambie, P. I. Higgs, P. S. Rutledge, and P. D. Woodgate, Aust. J. Chem., 47, 1815 (1994).
N. Kakusawa, K. Sakamoto, J. Kurita, and T. Tsuchiya, Heterocycles, 43, 2091 (1996).
K. Matsumoto, T. Uchida, K. Aoyama, M. Nishikawa, T. Kuroda, and T. Okamoto, J. Heterocycl. Chem., 25, 1793 (1988).
M. G. Reinecke, Tetrahedron, 38, 427 (1982).
S. P. Khanapure, B. M. Bhawal, and E. R. Biehl, Heterocycles, 32, 1773 (1991).
N. Kakusawa, M. Imamura, J. Kurita, and T. Tsuchiya, Heterocycles, 38, 957 (1994).
N. D. Rondan, N. L. Domelsmith, K. N. Houk, A. T. Bowne, and R. H. Levin, Tetrahedron Lett., 35, 3237 (1979).
J. M. Caroon and L. E. Fisher, Heterocycles, 32, 459 (1991).
S. Tandel, A. Wang, T. C. Holdeman, H. Zhang, and E. R. Biehl, Tetrahedron, 54, 15147 (1998).
A. R. Deshmukh and E. R. Biehl, Heterocycles, 34, 99 (1992).
A. Wang and E. Biehl, J. Chem. Soc., Perkin Trans. 1, 1461 (1998).
A. Wang, H. Zhang, and E. R. Biehl, Heterocycles, 52, 1133 (2000).
Z. Liu and R. C. Larock, Org. Lett., 6, 3739 (2004).
Z. Liu and R. C. Larock, Tetrahedron, 63, 347 (2007).
H. Yoshida, S. Sugiura, and A. Kunai, Org. Lett., 4, 2767 (2002).
J. Zhao and R. C. Larock, Org. Lett., 7, 4273 (2005).
J. Barluenga, F. J. Fananas, R. Sanz, and Y. Fernandez, Chem.–Eur. J., 8, 2034 (2002).
A. M. d'A. Rocha Gonsalves, T. M. V. D. P. Melo, and T. L. Gilchrist, Tetrahedron, 48, 6821 (1992).
R. Dhar, W. Huhnermann, T. Kampchen, W. Overheu, and G. Seitz, Chem. Ber., 116, 97 (1983).
S. Diring, P. Retailleau, and R. Ziessel, Tetrahedron Lett., 48, 8069 (2007).
S. Diring, P. Retailleau, and R. Ziessel, J. Org. Chem., 72, 10181 (2007).
M. Girardot, R. Nomak, and J. K. Snyder, J. Org. Chem., 63, 10063 (1998).
D. Margetic, Y. Murata, K. Komatsu, and Z. Marinic, Helv. Chim. Acta, 92, 298 (2009).
S. C. Benson, J. L. Gross, and J. K. Snyder, J. Org. Chem., 55, 3257 (1990).
G. Seitz, R. Hoferichter, and R. Mohr, Angew. Chem., 99, 345 (1987).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 4, pp. 576-587, April, 2012.
Rights and permissions
About this article
Cite this article
Kovalev, I.S., Kopchuk, D.S., Zyryanov, G.V. et al. Aryne intermediates in the synthesis of polynuclear heterocyclic systems (Review). Chem Heterocycl Comp 48, 536–547 (2012). https://doi.org/10.1007/s10593-012-1028-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-012-1028-9