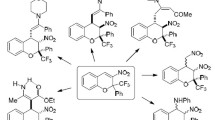
Recent literature data for the reaction of 3-cyano(thio)chromones with amines, hydrazines, and hydroxylamine are summarized.
Similar content being viewed by others
References
C. K. Ghosh and S. K. Karak, J. Heterocycl. Chem., 42, 1035 (2005).
A. Nohara, T. Ishiguro, K. Ukawa, H. Sugihara, Y. Maki, and Y. Sanno, J. Med. Chem., 28, 559 (1985).
C. K. Ghosh and N. Tewari, J. Org. Chem., 45, 1964 (1980).
U. Petersen and H. Heitzer, Liebigs Ann. Chem., 1659 (1976).
C. K. Ghosh, Synth. Commun., 8, 487 (1978).
C. K. Ghosh, C. Ghosh, and A. Patra, Indian J. Chem., 37B, 387 (1998).
V. Y. Sosnovskikh, V. S. Moshkin, and M. I. Kodess, Tetrahedron Lett., 50, 6515 (2009).
V. Y. Sosnovskikh, V. S. Moshkin, and M. I. Kodess, Izv. Akad. Nauk, Ser. Khim., 602 (2010).
F. Risitano, G. Grassi, and F. Foti, J. Heterocycl. Chem., 38, 1083 (2001).
I. Sigg, G. Haas, and T. Winkler, Helv. Chim. Acta, 65, 275 (1982).
G. Rihs, I. Sigg, G. Haas, and T. Winkler, Helv. Chim. Acta, 68, 1933 (1985).
M. Abdel-Megid, Khim. Geterotsikl. Soedin., 1888 (2009). [Chem. Heterocycl. Compd., 45, 1523 (2009)].
V. Y. Sosnovskikh, V. S. Moskhin, and O. S. El'tsov, Izv. Akad. Nauk, Ser. Khim., 2097 (2010).
C. K. Ghosh, N. Tewari, and C. Bandyopadhyay, Indian J. Chem., 22B, 1200 (1983).
J. Dusemund and T. Schurreit, Arch. Pharm., 317, 377 (1984).
V. Y. Sosnovskikh, D. V. Sevenard, V. S. Moshkin, V. O. Iaroshenko, and P. Langer, Tetrahedron, 66, 7322 (2010).
V. Y. Sosnovskikh, D. V. Sevenard, V. S. Moshkin, and O. S. El'tsov, Izv. Akad. Nauk, Ser. Khim., 2101 (2010).
C. K. Ghosh, D. K. SinhaRoy, and K. K. Mukhopadhyay, J. Chem. Soc., Perkin Trans. 1, 1964 (1979).
V. Y. Sosnovskikh, V. S. Moshkin, and M. I. Kodess, J. Heterocycl. Chem., 47, 629 (2010).
B. Chantegrel, A.-I. Nadi, and S. Gelin, Tetrahedron Lett., 24, 381 (1983).
I. Strakova, M. Petrova, S. Belyakov, and A. Strakov, Khim. Geterotsikl. Soedin., 1827 (2003). [Chem. Heterocycl. Compd., 39, 1608 (2003)].
T. Steinführer, A. Hantschmann, M. Pietsch, and M. Weissenfels, Liebigs Ann. Chem., 23 (1992).
Z. Jerzmanowska, W. Basiński, and L. Zielińska, Polish J. Chem., 54, 383 (1980).
W. Basiński and Z. Jerzmanowska, Polish J. Chem., 57, 471 (1983).
A. Nohara, H. Sugihara, and K. Ukawa, JP Pat. Appl. 53111071 (1978); Chem. Abstr., 90, 54827 (1979).
V. Y. Sosnovskikh, V. S. Moshkin, and M. I. Kodess, Tetrahedron Lett., 49, 6856 (2008).
V. Y. Sosnovskikh and V. S. Moshkin, Izv. Akad. Nauk, Ser. Khim., 1031 (2010).
V. Y. Sosnovskikh, M. I. Kodess, and V. S. Moshkin, Izv. Akad. Nauk, Ser. Khim., 1218 (2009).
V. Y. Sosnovskikh, V. S. Moshkin, and M. I. Kodess, Mendeleev Commun., 20, 209 (2010).
V. Y. Sosnovskikh, V. S. Moshkin, M. Y. Kornev, and M. I. Kodess, Mendeleev Commun., 21, 110 (2011).
T. Zarganes-Tzitzikas, M. A. Terzidis, J. Stephanidou-Stephanatou, C. A. Tsoleridis, and G. E. Kostakis, J. Org. Chem., 76, 9008 (2011).
M. F. Ibad, O.-R. Abid, M. Adeel, M. Nawaz, V. Wolf, A. Villinger, and P. Langer, J. Org. Chem., 75, 8315 (2010).
M. A. Rashid, N. Rasool, B. Appel, M. Adeel, V. Karapetyan, S. Mkrtchyan, H. Reinke, C. Fischer, and P. Langer, Tetrahedron, 64, 5416 (2008).
P. Langer, Synlett, 1016 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 1, pp. 144-152, January, 2012.
Rights and permissions
About this article
Cite this article
Sosnovskikh, V.Y., Moshkin, V.S. Novel data for the reaction of 3-cyano-(thio)chromones with N-nucleophiles. Chem Heterocycl Comp 48, 139–146 (2012). https://doi.org/10.1007/s10593-012-0977-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-012-0977-3