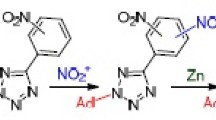
For the case of 1-(allylsulfanyl)-2-methoxy-N-(1-methylethylidene)buta-1,3-dien-1-amine, obtained from α-lithiated methoxyallene, isopropyl isothiocyanate, and allyl bromide, it was shown that 5-ethylidene-2-(1-methoxyprop-2-enyl)-4,4-dimethyl-4,5-dihydro-1,3-thiazole and 6-methoxy-2-methyl-3H-azepine are formed competitively upon the action of the t-BuOK–THF–DMSO system.


Similar content being viewed by others
References
G. R. Proctor and J. Redpath (editors), Chemistry of Heterocyclic Compounds. Vol. 56. Monocyclic Azepines: The Synthesis and Chemical Properties of the Monocyclic Azepines, Wiley, Chichester, New York, 1996.
R. K. Smalley, in: A. R. Katritzky and C. W. Rees (editors), Comprehensive Heterocyclic Chemistry, Elsevier, Oxford, 1984, vol. 7, p. 491.
D. J. Le Count, in: A. R. Katritzky, C. W. Rees, and E. F. V. Scriven (editors), Comprehensive Heterocyclic Chemistry II, Elsevier, Oxford, 1996, vol. 9, p. 1.
J. B. Bremner, S. Samosorn, in: A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven, and R. J. K. Taylor (editors), Comprehensive Heterocyclic Chemistry III, Elsevier, Oxford, 2008, vol. 13, p. 1.
Y. Kubota, K. Satake, R. Ikui, H. Okamoto, and M. Kimura, Bull. Chem. Soc. Jpn., 76, 805 (2003).
J. H. Rigby and F. C. Pigge, J. Org. Chem., 60, 7392 (1995).
M. Decker and J. Lehmann, Arch. Pharm., 336, 466 (2003).
S. Superchi, E. Giorgio, P. Scafato, and C. Rosini, Tetrahedron: Asymmetry, 13, 1385 (2002).
V. Bisai and V. K. Singh, Synlett, 481 (2011).
T. Ooi, Y. Uematsu, M. Kameda, and K. Maruoka, Tetrahedron, 62, 11425 (2006).
N. A. Nedolya, L. L. Dmitrieva, A. I. Albanov, L. V. Klyba, O. A. Tarasova, and I. A. Ushakov, Zh. Org. Khim., 42, 465 (2006).
N. A. Nedolya, O. A. Tarasova, O. G. Volostnykh, A. I. Albanov, and B. A. Trofimov, J. Organomet. Chem., 696, 3359 (2011).
N. A. Nedolya, Novel Chemistry Based on Isothiocyanates and Polar Organometallics, Thesis, Utrecht University, Utrecht, The Netherlands, 1999.
L. Brandsma, Eur. J. Org. Chem., 4569 (2001).
L. Brandsma and N. A. Nedolya, Synthesis, 735 (2004).
N. A. Nedolya, Khim. Geterotsikl. Soedin., 1443 (2008). [Chem. Heterocycl. Comp., 44, 1165 (2008)].
L. Brandsma, Preparative Polar Organometallic Chemistry, Springer-Verlag, Berlin, 1990, Vol. 2, p. 108.
M. Piffl, J. Weston, W. Günther, and E. Anders, J. Org. Chem., 65, 5942 (2000).
H. J. Reich and W. W. Willis, Jr., J. Org. Chem., 45, 5227 (1980).
D. S. Tarbell and M. A. McCall, J. Am. Chem. Soc., 74, 48 (1952).
C. C. Price and W. H. Snyder, J. Org. Chem., 27, 4639 (1962).
D. S. Tarbell and W. E. Lovett, J. Am. Chem. Soc., 78, 2259 (1956).
S. Hoff, L. Brandsma, and J. F. Arens, Recl. Trav. Chim. Pays-Bas, 87, 916 (1968).
B. A. Trofimov, N. A. Nedolya, V. V. Gerasimova, and M. G. Voronkov, Sulfur Lett., 8, 73 (1988).
Author information
Authors and Affiliations
Corresponding author
Additional information
*Dedicated to M. G. Voronkov on his 90th birthday.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 11, pp. 1718-1724, November, 2011.
Rights and permissions
About this article
Cite this article
Nedolya, N.A., Tarasova, O.A., Volostnykh, O.G. et al. Reactions of 2-aza-1,3,5-trienes with superbases: competition between formation of thiazoles and azepines. Chem Heterocycl Comp 47, 1430–1435 (2012). https://doi.org/10.1007/s10593-012-0930-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-012-0930-5