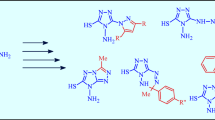
A simple method has been developed for the synthesis of water-soluble pyrazole derivatives, namely 4-[bis(2-hydroxyethylsulfanyl)methyl]pyrazoles hydrochlorides, by the reaction of a series of pyrazole carbaldehydes with 2-mercaptoethanol in the presence of trimethylchlorosilane. When treated with aqueous ammonia solution the pyrazole-4-carbaldehydes bis(2-hydroxyethyl)dithioacetal hydro-chlorides are converted to the 4-[bis(2-hydroxyethylsulfanyl)methyl]pyrazole free bases.




Similar content being viewed by others
References
T. W. Greene and P. G. M. Wuts, Protective Groups in Organic Synthesis, 2nd Edition, Wiley, New York (1991).
S. Oh, I. H. Jeong, C. M. Ahn, V. S. Shin, and S. Lee, Bioorg. Med. Chem., 12, 3783 (2004).
K. Benda, W. Regenhardt, E. Shaumann, and G. Adiwidjaja, Eur. J. Org. Chem., 1016 (2009).
K. C. Golden, B. T. Gregg, and J. F. Quinn, Tetrahedron Lett., 51, 4010 (2010).
T. Mori, Y. Sawada, and A. Oku, J. Org. Chem., 65, 3620 (2000).
M. Ioannou, M. J. Porter, and F. Saez, Chem. Commun., 346 (2002).
K. Krohn and S. Cludius-Brandt, Synthesis, 1344 (2010).
K. Utimoto, A. Nakamura, and S. Matsubara, J. Am. Chem. Soc., 112, 8189 (1990).
J. E. Lynch and E. L. Eliel, J. Am. Chem. Soc., 106, 2943 (1984).
E. L. Eliel and S. Morris-Natschke, J. Am. Chem. Soc., 106, 2937 (1984).
S. Dei, P. Angeli, C. Bellucci, M. Buccioni, F. Gualtieri, G. Marrucci, D. Manetti, R. Matucci, M. N. Romanelli, S. Scapecchi, and E. Teodori, Biochem. Pharmacol., 69, 1637 (2005).
P. C. Bulman Page, M. B. van Niel, and J. C. Prodger, Tetrahedron, 45, 7643 (1989).
A. T. Khan, E. Mondal, S. Ghosh, and S. Islam, Eur. J. Org. Chem., 2002 (2004).
S. Ter-Sarkisyan, Izv. Akad. Nauk SSSR, Ser. Khim., 1988 (1960).
C. Macleod, G. J. McKiernan, E. J. Guthrie, L. J. Farrugia, D. W. Hamprecht, J. Macritchie, and R. C. Hartley, J. Org. Chem., 68, 387 (2003).
B. Karimi, H. Seradj, and J. Maleki, Tetrahedron, 58, 4513 (2002).
K. Krohn and S. Cludius-Brandt, Synthesis, 2369 (2008).
Y. Wang, G. Inguaggiato, M. Jasamai, M. Shah, D. Hughes, M. Slater, and C. Simons, Bioorg. Med. Chem., 7, 481 (1999).
A. Martinez, A. I. Esteban, A. Herrero, C. Ochoa, G. Andrei, R. Snoeck, J. Balzarini, and E. De Clercq, Bioorg. Med. Chem., 7, 1617 (1999).
M. Camplo, A. S. Charvet-Faury, C. Borel, F. Turin, O. Hantz, V. Trabaud, C. Niddam, N. Mourier, J. C. Graciet, J. C. Chermann, and J. L. Kraus, Eur. J. Med. Chem., 31, 539 (1996).
J. W. Ralls, R. M. Dodson, and B. Riegel, J. Am. Chem. Soc., 71, 3320 (1949).
C. Djerassi and M. Gorman, J. Am. Chem. Soc., 75, 3704 (1953).
G. E. Wilson Jr., M. G. Huang, and W. W. Schloman Jr., J. Org. Chem., 33, 2133 (1968).
V. Kumar and S. Dev, Tetrahedron Lett., 24, 1289 (1983).
J. S. Yadav, B. V. S. Reddy, and S. K. Pandey, Synth. Commun., 32, 715 (2002).
V. K. Yadav and A. G. Fallis, Tetrahedron Lett., 29, 897 (1988).
E. Mondal, P. R. Sahu, G. Bose, and A. Khan, Tetrahedron Lett., 43, 2843 (2002).
B. Bogdan and Z. Kortylewicz, Synthesis, 831 (1982).
K. Manabe, S. Iimura, X-M. Sun, and S. Kobayashi, J. Am. Chem. Soc., 124, 11971 (2002).
A. Kumar, M. S. Rao, and V. K. Rao, Aust. J. Chem., 63, 135 (2010).
D. Azarifar and A. Forghaniha, J. Chin. Chem. Soc. (Taipei, Taiwan), 53, 1189 (2006).
P. Elguero, N. Goya, N. Jagerovic, and A. M. S. Silva, in: A. Attanasi and D. Spinelli (editors), Targets in Heterocyclic Systems. Chemistry and Properties, Vol. 6, Italian Chemical Society, Rome (2002), p. 53.
A. F. Grapov, Russ. Chem. Rev., 68, 697 (1999).
E. S. Domina, L. A. Es'kova, E. V. Petrova, N. N. Chipanina, V. K. Voronov, A. V. Afonin, and G. G. Skvortsova, Zh. Neorg. Khim., 32, 1523 (1987).
L. K. Papernaya, A. A. Shatrova, A. I. Albanov, E. V. Rudyakova, and G. G. Levkovskaya, Zh. Org. Khim., 47, 467 (2011).
L. K. Papernaya, A. A. Shatrova, A. I. Albanov, and G. G. Levkovskaya, Zh. Org. Khim., 47, 305 (2011).
Z.-Y. Peng, F.-F. Ma, L.-F. Zhu, X.-M. Xie, and Z. Zhang, J. Org. Chem., 74, 6855 (2009).
L. P. Turchaninova, N. A. Korchevin, A. T. Shipov, E. N. Deryagina, Yu. I. Baukov, and M. G. Voronkov, Zh. Obshch. Khim., 59, 722 (1989).
M. Kamiya, Bull. Chem. Soc. Jpn., 43, 3344 (1970).
I. A. Zyryanova, L. V. Baikalova, O. A. Tarasova, A. V. Afonin, V. A. Kukhareva, M. A. Maksimova, and B. A. Trofimov, Zh. Obshch. Khim., 75, 1353 (2005).
R. J. Pugmire and D. M. Grant, J. Am. Chem. Soc., 90, 4232 (1968).
I. I. Schuster and J. D. Roberts, J. Org. Chem., 44, 3864 (1979).
A. Pawer and A. A. Patil, Indian J. Chem. Sect. B, 33, 156 (1994).
A. F. Pozharskii, V. A. Anisimova, and E. B. Tsupak, Practical Work on Heterocyclic Chemistry [in Russian], Rostov on Don Publishing House (1988), p. 22.
E. V. Rudyakova, V. A. Savosik, L. K. Papernaya, A. I. Albanov, I. T. Evstaf'eva, and G. G. Levkovskaya, Zh. Org. Khim., 45, 1053 (2009).
This work was carried out with the financial support of the Russian Foundation for Fundamental Investigations (grant 10-03-00256a).
Author information
Authors and Affiliations
Corresponding author
Additional information
*Dedicated to Academician M. G. Voronkov on his 90th birthday.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 11, pp. 1680-1690, November, 2011.
Rights and permissions
About this article
Cite this article
Papernaya, L.K., Shatrova, A.A., Albanov, A.I. et al. Synthesis and properties of pyrazole carbaldehyde bis(2-hydroxyethyl)-dithioacetal hydrochlorides*. Chem Heterocycl Comp 47, 1395–1404 (2012). https://doi.org/10.1007/s10593-012-0927-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-012-0927-0