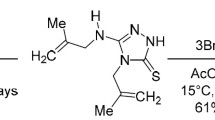
The behavior of imidazo[1,2-a]pyridinium, imidazo[1,2-a]pyrimidinium, and imidazo[2,1-b]thiazolium bromides derivatives in oxidative bromination reactions has been studied. It has been established that reaction products structures and their yields depend on the properties of the substituents in the bicycle and the oxidant concentration.
Similar content being viewed by others
Notes
The term imidazoheterocycles means bicyclic heterosystems, one of the rings of which is imidazole (for example, imidazopyridine, imidazopyrimidine, and imidazothiazole).
References
W. L. Mosby, Heterocyclic Systems with Bridgehead Nitrogen Atoms, Interscience, New York, Vol. 1, p. 239 (1961).
H. L. Blewitt in: A. Weissberger and E. C Taylor (editors), Special Topics in Heterocyclic Chemistry,Wiley, New York, p. 117 (1977).
E. E. Glover and M. Yorke, J. Chem. Soc. C, 3280 (1971).
T. Pyl, R. Giebelmann, and H. Beyer, Liebigs Ann. Chem., 643, 145 (1961).
N. O. Saldabol and Yu. Yu. Popelis, Khim. Geterotsikl. Soed., 691 (1972). [Chem. Heterocycl. Comp., 8, 627 (1972)].
S. C. Virgil in: L. A. Paquette (editor), Encyclopedia of Reagents for Organic Synthesis,,Wiley, Chichester, Vol. 1, p. 768 (1995).
N. O. Saldabol and O. E. Lando, Khim. Geterotsikl. Soed., 258 (1978). [Chem. Heterocycl. Comp., 14, 211 (1978)].
G. P. Kutrov, Yu. M. Volovenko, V. A. Kurg, E. N. Machkovskaya, and F. S. Babichev, Dokl. AN UkrSSR, Ser. B, 5, 37 (1989).
N. V. Kovalenko, G. P. Kutrov, O. V. Shishkin, S. V. Shishkina, and V. P. Khilya, Dokl. NAN Ukr., 10, 150 (2003).
G. P. Kutrov, N. V. Kovalenko, and Yu. P. Germanchuk, Ukr. Khim. Zh., 57, 187 (1991).
N. V. Kovalenko, G. P. Kutrov, Yu. D. Filipchuk, and M. Yu. Kornilov, Khim. Geterotiskl. Soed., 675 (2002). [Chem. Heterocycl. Comp., 38 , 590 (2002)].
A. F. Khlebnikov, G. I. Kostik, and R. R. Kostikov, Khim. Geterotiskl. Soed., 810 (1991). [Chem. Heterocycl. Comp., 27 , 636 (1991)].
J. P. Wibaut and G. M. Kraay, Recl. Trav. Chim. Pays Bas, 42, 1084 (1923).
M. A. O’Daly, C. P. Hopkinson, G. D. Meakins, and A. J. Raybould, J. Chem. Soc., Perkin Trans. 1, 855 (1991).
G. M. Sheldrick, Acta Crystallorg., A64, 112 (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
*Dedicated to the illustrious life of Academician M.O. Lozinskii.
Deceased. (G. P. Kutrov)
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No.10, 1548-1554, October, 2011.
Rights and permissions
About this article
Cite this article
Kovalenko, N.V., Cherepakha, A.Y., Kutrov, G.P. et al. Oxidative bromination of imidazoheterocycle bromohydrates*. Chem Heterocycl Comp 47, 1280–1285 (2012). https://doi.org/10.1007/s10593-012-0903-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-012-0903-8