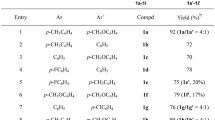
2-Phenacyl-1H-benzimidazoles were prepared by the acylation of 2-methylbenzimidazole using aroyl chlorides with subsequent alcoholysis or aminolysis of the N,C,O-triacylation products obtained. Treatment of the 2-phenacyl-1H-benzimidazoles with hydrobromic acid gave the corresponding salts. The structure of the products was supported by IR, 1H and 13 C NMR, and HMBC spectroscopy as well as by quantum-chemical calculations. 2-Phenacyl-1H-benzimidazoles in DMSO-d6 solution were found to display predominantly imino-enamino tautomerism, while their salts were found to display predominantly keto-enol tautomerism.

Similar content being viewed by others
References
A. A. Spasov, I. N. Iezhitsa, L. I. Bugaeva, and V. A. Anisimova, Khim.-farm. Zh., 33, No. 5, 6 (1999).
M. D. Mashkovskii, Drugs [in Russian], Novaya Volna, Moscow (2008), p. 622.
I. B. Dzvinchuk and M. O. Lozinskii, in: V. G. Kartsev (editor), Nitroge-containingn Heterocycles [in Russian], vol. 1, ICSPF, Moscow (2006), p. 275.
I. B. Dzvinchuk and M. O. Lozinskii, in: V. G. Kartsev (editor), Nitrogen-containing Heterocycles [in Russian], vol. 1, ICSPF, Moscow (2006), p. 281.
I. B. Dzvinchuk and M. O. Lozinskii, in: V. G. Kartsev (editor), Selected Methods for Synthesis and Modification of Heterocycles [in Russian], vol. 6, ICSPF, Moscow (2007).
A. Tajana, R. Pennini, and D. Nardi, Farmaco, Ed. Sci., 35, 181 (1980).
M. Sakamoto, M. Abe, and K. Ishii, Chem. Pharm. Bull., 39, 277 (1991).
V. P. Khilya, L. G. Grishko, and T. N. Sokolova, Khim. Geterotsikl. Soedin., 1593 (1975). [Chem. Heterocycl. Comp., 11, 1353 (1975)].
M. S. Frasinyuk, N. V. Gorbulenko, and V. P. Khilya, Khim. Geterotsikl. Soedin., 1237 (1997). [Chem. Heterocycl. Comp., 33, 1078 (1997)].
H.-J. Knolker, R. Bouse, and R. Hitzemann, Heterocycles, 29, 1551 (1989).
A. A. Macco, E. F. Godefroi, and J. J. M. Drouen, J. Org. Chem., 40, 252 (1975).
I. B. Dzvinchuk, M. O. Lozinskii, and A. V. Vypirailenko, Zh. Org. Khim., 30, 909 (1994).
Z.-T. Huang and M.-X. Wang, Synthesis, 1273 (1992).
Z.-T. Huang and M.-X. Wang, Tetrahedron, 2325 (1992).
I. B. Dzvinchuk, A. V. Gutov, A. N. Chernega, and M. O. Lozinskii, Khim. Geterotsikl. Soedin., 852 (2010). [Chem. Heterocycl. Comp., 46, 684 (2010)].
M. I. Kabachnik, Usp. Khim., 48, 1523 (1979).
A. Gordon and R. Ford, Chemist's Companion [Russian translation], Mir, Moscow (1976), pp. 293, 310.
V. Bekarek and J. Slouka, Collect. Czech. Chem. Commun., 35, 2936 (1970).
M. I. Rudnev, V. P. Kurbatov, N. K. Chub, and O. A. Osipov, Zh. Obshch. Khim., 58, 2334 (1988).
A. S. Nakhmanovich, T. N. Komarova, and Yu. A. Mansurov, Izv. Akad. Nauk, Ser. Khim., 1173 (1984).
J. Elguero, C. Marzin, A. R. Katritzky, and P. Linda, The Tautomerism of Heterocycles, Academic Press, New York-San Francisco-London (1976), pp. 190, 492.
A. D. Garnovskii and E. V. Sennikova, Khim. Geterotsikl. Soedin., 1603 (2007). [Chem. Heterocycl. Comp., 43, 1359 (2007)].
J. J. P. Stewart, MOPAC2009, Stewart Computational Chemistry, Version 9.069W web: http://OpenMOPAC.net.
A. Klamt and G. Schüürmann, J. Chem. Soc., Perkin Trans. 2, 799 (1993).
M. J. Frisch, G. V. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Chiseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yasiev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. J. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keich, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian03, revision B.05, Gaussian Inc., Pittsburgh, PA (2003).
J. B. Foresman and E. Frisch, Exploring Chemistry with Electronic Structure Methods, 2nd ed., Gaussian Inc., Pittsburgh, PA (2001).
Author information
Authors and Affiliations
Corresponding author
Additional information
*Deceased. (M. O. Lozinskii)
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1162–1174, August, 2011.
Rights and permissions
About this article
Cite this article
Dzvinchuk, I.B., Nesterenko, A.M., Polovinko, V.V. et al. Synthesis and tautomerism of 2-phenacyl-1H-benzimidazoles and their hydrogen bromide salts. Chem Heterocycl Comp 47, 953–963 (2011). https://doi.org/10.1007/s10593-011-0860-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-011-0860-7