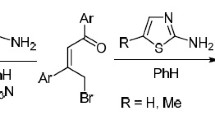
Fusion of 4-bromo-1,3-diphenyl-2-buten-1-ones (γ-bromodypnones) with 1,2-dimethyl-1H-benzimidazole and further treatment of the reaction product with a base (morpholine) gives 7,9-diaryl-5-methyl5,10-dihydroazepino[1,2-a]benzimidazol-11-ium bromides. The reaction of γ-bromodypnone with 1-alkyl-2-methyl-1H-imidazoles in benzene at 25 °C gives quaternary azolium salts. Upon heating their solutions in alcohol in the presence of K2CO3 the latter cyclize to 1-R-6,8-diaryl-1,5-dihydroimidazo[1,2-a]azepin-4-ium bromides or 1-R-6,8-diaryl-1H-imidazo[1,2-a]azepines depending on the nature of the substituent in the benzene rings and the substituent at the N(1) atom of the imidazole.



Similar content being viewed by others
References
A. Thurkauf, X. Chen, S. Zhang, Y. Gao, A. Kieltyka, J. W. F. Wasley, R. Brodbeck, W. Greenlee, A. Ganquly, and H. Zhao, Bioorg. Med. Chem. Lett., 13, 2921 (2003).
F. Novelli, B. Tasso, F. Sparatore, and A. Sparatore, Farmaco, 52, 499 (1997).
F. Janssens, J. Leenaerts, G. Diels, B. De Boeck, A. Megens, X. Langlois, K. van Rossem, J. Beetens, and M. Borgers, J. Med. Chem., 48, 2154 (2005).
J. M. Elliott, E. J. Carlson, G. G. Chicchi, O. Dirat, M. Dominguez, U. Gerhard, R. Jelley, A. B. Jones, M. M. Kurtz, K. lan Tsaoc, and A. Wheeldon, Bioorg. Med. Chem. Lett., 16, 2929 (2006).
F. Piu, N. K. Gauthier, R. Olsson, E. A. Currier, B. W. Lund, G. E. Croston, U. Hacksell, and M. R. Brann, Biochem. Pharmacol., 71, 156 (2005).
V. A. Kovtunenko, L. M. Potikha, A. R. Turelyk, and A. V. Turov, Khim. Geterotsikl. Soedin., 791 (2008). [Chem. Heterocycl. Comp., 44, 632 (2008)].
L. M. Potikha, A. R. Turelyk, V. A. Kovtunenko, and A. V. Turov, Khim. Geterotsikl. Soedin., 95 (2010). [Chem. Heterocycl. Comp., 46, 82 (2010)].
D. A. Filimonov, V. V. Poroikov, Yu. V. Borodina, and T. Gloriozova, J. Chem. Inf. Comput. Sci., 39, 666 (1999).
V. V. Poroikov, D. A. Filimonov, Yu. V. Borodina, A. A. Lagunin, and A. Kos, J. Chem. Inf. Comput. Sci., 40, 1349 (2000).
V. V. Poroikov and D. A. Filimonov, J. Comput.-Aided Mol. Des., 16, 819 (2002).
M. Node, S. Kodama, Y. Hamashima, T. Katoh, K. Nishide, and T. Kajimoto, Chem. Pharm. Bull., 54, 1662 (2006).
C.-H. Chou, L.-T. Chu, I-Y. Chen, and B.-J. Wu, Heterocycles, 75, 577 (2008).
V. Gracias, A. F. Gasiecki, T. G. Pagano, and S. W. Djuric, Tetrahedron Lett., 47, 8873 (2006).
H. S. Lee, S. H. Kim, S. Gowrisankar, and J. N. Kim, Tetrahedron, 64, 7183 (2008).
J. R. McClure, J. H. Custer, H. D. Schwarz, and D. A. Lill, Synlett., 710 (2000).
S. Ohta, Y. Narita, T. Yuasa, S. Hatakeyama, M. Kobayashi, K. Kaibe, I. Kawasaki, and M. Yamashita, Chem. Pharm. Bull., 39, 2787 (1991).
S. Ohta, Y. Narita, M. Okamoto, S. Hatakeyama, K. Kan, T. Yuasa, and K. Hayakawa, Chem. Pharm. Bull., 38, 301 (1990).
L. M. Potikha, A. R. Turelyk, and V. A. Kovtunenko, Khim. Geterotsikl. Soedin., 1478 (2009). [Chem. Heterocycl. Comp., 45, 1184 (2009)].
A. Fozard and G. Jones, J. Org. Chem., 30, 1523 (1965).
R. M. Acheson and W. R. Tully, J. Chem. Soc. (C), 1623 (1968).
H. H. Wassermann and N. E. Aubrey, J. Am. Chem. Soc., 75, 96 (1953).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 6, pp. 901–912, June 2011.
Rights and permissions
About this article
Cite this article
Potikha, L.M., Turelyk, A.R. & Kovtunenko, V.A. Synthesis of azepino[1,2-a]benzimidazoles and imidazo[1,2-a]azepines. Chem Heterocycl Comp 47, 745–754 (2011). https://doi.org/10.1007/s10593-011-0829-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-011-0829-6