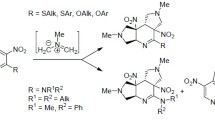
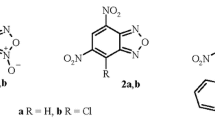
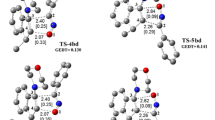
Synthetic approaches to 1,3-dipolar cycloaddition of N-methylazomethinylid to mononitrobenzazole are described. The geometric and electronic structures have been studied by quantum chemical methods (HF/STO-3 G and B3LYP/6-31 G*) and the reactivity indexes of compounds have been estimated. It was shown that 1,3-dipolar cycloaddition of N-methylazomethinylid to the dipolarophile has a polar character and proceeds in accordance with the normal (noninversion) electronic distribution.



Similar content being viewed by others
References
A. Padwa and W. H. Pearson, Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry To Heterocycles and Natural Products, Wiley, New York (2002), p. 169.
S. Roy, T. L. S. Kishbaugh, J. P. Jasinski, and G. W. Gribble, Tetrahedron Lett., 48, 1313 (2007).
R. Grigg and M. A. B. Sarker, Tetrahedron, 62, 10332 (2006).
B. F. Bonini, F. Boschi, M. C. Franchini, M. Fochi, F. Fini, A. Mazzanti, and A. Ricci, Synlett, 543 (2006).
C. Najera and J. M. Sansano, Curr. Org. Chem., 7, 1105 (2003).
M. Ghandi, A. Taheri, and A. Abbasi, J. Heterocycl. Chem., 47, 611 (2010).
J. M. Longmire, B. Wang, and X. Zhang, J. Am. Chem. Soc., 124, 13400 (2002).
C. Alemparte, G. Blay, and K. A. Jorgensen, Org. Lett., 7, 4569 (2005).
J. Xie, K. Yoshida, K. Takasu, and Y. Takemoto, Tetrahedron Lett., 49, 6910 (2008).
M.-X. Xue, X.-M. Zhang, and L.-Z. Gong, Synlett, 691 (2008).
M. A. Bastrakov, A. M. Starosotnikov, S. Yu. Pechenkin, V. V. Kachala, I. V. Glukhov, and S. A. Shevelev, J. Heterocycl. Chem., 47, 893 (2010).
O. Tsuge and S. Kanemasa, Adv. Heterocycl. Chem., 45, 231 (1989).
A. Viryani, G. Marth, A. Dancso, G. Blasko, L. Toke, and M. Nyerges, Tetrahedron, 62, 8720 (2006).
A. M. Starosotnikov, M. A. Bastrakov, S. Yu. Pechenkin, M. A. Leontieva, V. V. Kachala, and S. A. Shevelev, J. Heterocycl. Chem., (2011). (in the press: DOI 10.1002/jhet.599).
P. B. Ghosh and M. W. Whitehouse, J. Med. Chem., 11, 305 (1968).
T. Murashima, D. Shiga, K. Nishi, H. Uno, and N. Uno, J. Chem. Soc., Perkin Trans. 1, 2671 (2000).
H. G. Garg, J. Org. Chem., 27, 3683 (1962).
T. Murashima, K. Fujita, K. Ono, T. Ogawa, H. Uno, and N. Ono, J. Chem. Soc., Perkin Trans. 1, 1403 (1996).
A. M. Starosotnikov and S. A. Shevelev, Izv. Akad. Nauk, Ser. Khim., 1703 (2003).
V. M. Vinogradov, A. M. Starosotnikov, and S. A. Shevelev, Mendeleev Commun., 198 (2002).
A. M. Starosotnikov, A. V. Lobach, Yu. A. Khomutova, and S. A. Shevelev, Izv. Akad. Nauk, Ser. Khim., 523 (2006).
A. M. Starosotnikov, V. V. Kachala, A. V. Lobach, V. M. Vinogradov, and S. A. Shevelev, Izv. Akad. Nauk, Ser. Khim., 1690 (2003).
V. M. Vinogradov, I. L. Dalinger, A. M. Starosotnikov, and S. A. Shevelev, Izv. Akad. Nauk, Ser. Khim., 445 (2001).
M. J. Frish, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery Jr., R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, A. G. Baboul, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komazomi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzales, M. Head-Gordon, E. S. Replogle, and J. A. Pople, GAUSSIAN 98. Revision A.9, Gaussian Inc., Pittsburgh PA (1998).
R. G. Parr, L. V. Szentpaly, and S. Liu, J. Am. Chem. Soc., 121, 1922 (1999).
R. G. Parr and W. Yang, Density Functional Theory of Atoms and Molecules, Oxford Univ. Press, New York (1989).
L. R. Domingo, M. Arno, R. Contreras, and P. Perez, J. Phys. Chem. A, 106, 952 (2002).
R. Sustmann, Tetrahedron Lett., 12, 2717 (1971).
R. Sustmann, Pure Appl. Chem., 40, 569 (1974).
M. L. Kuznetsov, Usp. Khim., 75, 1045 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to splendid chemist and remarkable person, meritorious scientist of the Russian Federation, Professor Leonid Isaakovich Belen'kii, on his 80th birthday.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 2, pp. 271–278, February, 2011.
Rights and permissions
About this article
Cite this article
Starosotnikov, A.M., Khakimo, D.V., Bastrakov, M.A. et al. Special features of 1,3-dipolar cycloaddition of n-methylazomethinylid to nitrobenzazoles. Chem Heterocycl Comp 47, 215–221 (2011). https://doi.org/10.1007/s10593-011-0743-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-011-0743-y