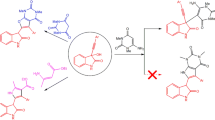
Some bifunctional heterocyclic systems having vicinal chlorocyano, chloroacetyl, and chloro-ethoxycarbonyl groups in their structures reacted with 6-amino-1,3-dimethyluracil to afford novel triheterocyclic systems having a pyrimidinedione moiety. Enaminones and α-cyanocinnamic acid derivatives in this reaction gave pyrido[2,3-d]pyrimidinediones. The antimicrobial activity of some new synthesized triheterocyclic systems was studied.





Similar content being viewed by others
References
A. Gangjee, O. Aldair, and S. F.Queener, J. Med. Chem., 42, 2447 (1999).
Michael D. Varney, Cynthial L. Palmer, and Williams H. Romines, PCT Int. Appl. WO 94 17076, US Pat. Appl. 1086129 (1993); Chem. Abstr., 121, 205385 (1994).
S. Furuya and T. Ohtaki, Eur. Pat. Appl. EP 608565 (1994); Chem. Abstr., 121, 205395 (1994).
D. Haber, C. Heers, and U. Ravens, Pharmazie, 48, 537 (1993).
G. Singh, A. K. Yadav, and K. Mishraa, Indian J. Chem., 41, 430 (2002).
N. R. Mohamed, M. T. Elsaidi, Y. M. Ali, and M. H. Elnagdi, Bioorg., Med. Chem., 41, 430 (2002).
Y. Sakuma, M. Hasegawa, K. Kataoka, K. Hoshina, N. Yamazaki, T. Kadota, and H. Yamaguchi, PCT Int. Appl. WO 91 05785; Chem. Abstr., 115, 71646 (1991).
J. I. De-Graw, P. H. Christie, W. T. Clowell, and F. Sirotnak, J. Med. Chem., 35, 320 (1992).
E. Lunt, C. C. Newton, in: R. Katritzky, C. W. Rees (editors), Comprehensive Heterocyclic Chemistry, Pergamon Press, Oxford, 1984, Vol. 3, p. 199.
H. Wamhoff, J. Dzenis, and K. Hirota, Adv. Heterocycl. Chem., 55, 129 (1992).
J. Qurroga, J.Garcia, B. Insuasty, N. L. Mendoza, M. Pungo, and H. Meier, H. Ann. Quim., 90, 300 (1994).
J. Qurroga, J. Garcia, B. Insuasty, M. Nogueras, and H. Mier, J. Heterocycl. Chem., 29, 1045 (1992).
J. Qurroga, A. Hormaza, B. Insuasty, M. Nogueras, A. Sanchez, N. Hanold, and H. Mier, J. Heterocycl. Chem., 34, 521 (1997).
J. Bulic, Bioorg. Med. Chem., 14, 2837 (2006).
K. Hirota, H. Kuki, and Y. Maki, Heterocycles, 37, 163 (1994).
P. Srivastava, A. S. Saxena, and V. J. Ram, Synthesis, 541 (2000).
A. D. Room, J. I. Shim, and G. L. Anderson, J. Org. Chem., 41, 1095 (1976).
M. Abdel-Megid, Pharmazie, 55, 263 (2006).
M. Abdel-Megid, Synth. Commmun., 37, 3211 (2007).
M. Abdel-Megid, Int. J. Chem., 16, 149 (2006).
M. Abdel-Megid, Heterocycl. Commmun., 4, 235 (1998).
M. Seada, M. Fawazy, H. Jahine, M. Abdel-Megid, and R. R Saad, J. Chin. Chem. Soc., 36, 241 (1989).
J. Quiroga, B. Insusaty, H. Insusty, and R. Absenes, J. Heterocycl. Chem., 38, 339 (2001).
M. Abdel-Megid, M. H. Elnagdi, and A. M. Negm, J. Heterocycl. Chem., 39, 105 (2003).
M. Abdel-Megid, Synth. Commmun., 33, 153 (2003).
M. Abdel-Megid, M. Abass, and M. H. Hassan, J. Heterocycl. Chem., 44, 315 (2007).
M. Abdel-Megid, Int. J. Chem., 16, 149 (2006).
K. Hirota, Y. Kitade, and S. Senda, J. Heterocycl. Chem., 22, 345 (1985).
Y. Tominaga, H. Oknda, Y. Mitsutomi, Y. Mastuda, G. Kobayashi, and K. Sakemi, Heterocycles, 12, 503 (1979).
E. F.Schoeder and R. M. Dadsom, J. Am. Chem. Soc., 84, 1904 (1962).
W. Pfleiderer, K. H. Schundehutte, and H. Ferch, Liebigs Ann. Chem., 615, 57 (1958).
J. C. Vincent and H. W. Vincent, Proc. Soc. Exp. Biol. Med., 55, 162 (1964).
M. Seada, M. Abdel-Megid, and I. M. El-Deen, Ind. J. Heterocycl. Chem., 3, 81 (1993).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 3, pp. 405-414, March, 2010.
Rights and permissions
About this article
Cite this article
Abdel-Megid, M. A convenient route for the synthesis of some new bi- and triheterocondensed uracils. Chem Heterocycl Comp 46, 316–324 (2010). https://doi.org/10.1007/s10593-010-0507-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-010-0507-0