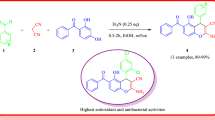
2-(p-Acetylaminobenzenesulfonylamido)-substituted benzothiazoles were prepared from 2-amino-substituted benzothiazoles and p-acetamidobenzenesulfonyl chloride using a mixture of pyridine and Ac2O, which formed an electrophilic N-acetyl- pyridinium complex facilitating condensation to give the desired products by removal of HCl. 2-[4-(Substituted benzothiazol-2-yl)aminosulfonylanilino]pyridine-3-carboxylic acids (synthesized from 2-chloropyridine-3-carboxylic acid and the corresponding substituted 2-(p-aminobenzenesulfonylamido)benzothiazole in 2-ethoxyethanol using Cu-powder and K2CO3) were then converted to acid chlorides, which on further reaction with piperazine and 4-methoxyphenylpiperazine yielded the corresponding 2-[4-(substituted benzothiazol-2-yl)amino-sulfonyl]anilino-3-(piperazinocarbonyl) pyridine and 2-[4-(substituted benzothiazol-2-yl)amino-sulfonyl]anilino-3-[(4-methoxyphenyl)piperazin-1-yl-carbonyl]pyridine. The structures of the new compounds have been established on the basis of their elemental analyses as well as IR, 1H NMR, and mass-spectral data. All the compounds have been screened for antimicrobial activity and found to possess considerable antibacterial activity.
Similar content being viewed by others
References
P. D. Bouzard, P. D. Cesare, C. Dussy, J. P. Jacquet, and A. Jaegly, J. Heterocycl. Chem., 29, 985 (1992).
O. Tabarrini, G. Manfroni, A. Fravolini, V. Cecchetti, S. Stefano, E. D. Clereq, J. Rozenski, B. Canard, H. Dutarte, J. Paeshuyse, and J. Neyts, J. Med. Chem., 49, 2621 (2006).
S. X. Cai, N. S. Jia, J. Herich, J. Guastella, S. Reddy, B. Tseng, J. Drewe, and S. Kasibhatla, J. Med. Chem., 46, 2474 (2003).
J. A. Lowe, Austrian Pat. 388378; Chem. Abstr., 112, 21008 (1990).
B. M. Gurupadaiah, E. Jayachandran, B. ShivaKumar, A. N. Nagappa, and L. V. G. Nargund, Indian J. Heterocycl. Chem., 7, 213 (1998).
P. Gopkumar, B. Shivakumar, E. Jayachandran, A. N. Nagappa, L. V. G. Nargund, and B. M. Gurupadaiah, Indian J. Heterocycl. Chem., 11, 39 (2001).
B. G. Khadse and S. R. Sengupta, Indian J. Chem., 32B, 407 (1993).
S. I. Ratzne, D. Bazsing, I. Jakozi, L. G. Kovanyine, G. Blasko, G. Siming, I. Gacsalyi, and E. Schmidt, PCT Int. Appl. WO 97 44334 (1997); Chem. Abstr., 128, 34786 (1998).
R. C. Trpathi, P. R. Dua, R. C. Srimal, and A. K. Saxena, Indian J. Chem., 34B, 116 (1995).
K. Araki, T. Kuroda, S. Uemori, A. Morigucsi, Y. Ikeda, F. Hirayama, Y. Yokoyama, E. Iwao, and T. Yakushiji, J. Med. Chem., 36, 1356 (1993).
S. Seshadri, N. M. Sanghavi, K. V. Naik, S. K. Tawate, M. N. Trivedi, and M. A. Fruitwala, Indian J. Chem., 32B, 688 (1993).
N. B. Patel and S. N. Agravat, Indian J. Heterocycl. Chem., 15, 395 (2006).
N. B. Patel and S. N. Agravat, Oriental J. Chem., 22, 333 (2006).
N. B. Patel and S. N. Agravat, J. Saudi Chem. Soc., 10, 373 (2006).
N. B. Patel and P. R. Bhagat, J. Indian Coun. Chem., 18, 56 (2001).
A. L. Barry, The Antimicrobial Susceptibility Test; Principles and Practices, Lea and Febiger, Philadelphia, Pa, USA, 1976.
S. R. Bhusare, R. P. Pawar, and Y. B. Vibhute, Indian J. Heterocycl. Chem., 11, 79 (2001).
A. I. Vogel, Small Scale Preparations, CBS Publ., Distributors, Delhi, 2nd ed., 1998, Pt I, p. 366.
J. A. Lowe III, R. L. Archer, D. S. Chapin, J. B. Cheng, D. Helweg, J. L. Johnson, B. K. Koe, L. A. Lebel, P. F. Moore, J. A. Nielsen, L. L. Russo, and J. T. Shirley, J. Med. Chem., 34, 624 (1991).
A. I. Vogel, Small Scale Preparations, CBS Publ., Distributors, Delhi, 2nd ed., 1998, Pt II, p. 209.
M. Vlassa, J. Barbe, and P. Brounat, Indian J. Heterocycl. Chem., 7, 149 (1997).
A. I. Vogel, Small Scale Preparations, CBS Publ., Distributors, Delhi, 2nd ed., 1998, Pt I, p. 345.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 11, pp. 1672–1683, November, 2009.
Rights and permissions
About this article
Cite this article
Patel, N.B., Agravat, S.N. Synthesis and antimicrobial studies of new pyridine derivatives. Chem Heterocycl Comp 45, 1343–1353 (2009). https://doi.org/10.1007/s10593-010-0432-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-010-0432-2