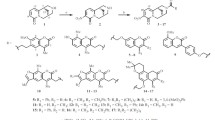
The interaction of 4-hydroxycoumarin with phenyl-, 2-chlorophenyl- and 4-bromophenyhydrazine hydrochlorides in the presence of triethylamine led in all cases to the corresponding 4-(arylhydrazino)-coumarins and 1-aryl-3-(2-hydroxyphenyl)-2H-pyrazolin-5-ones. 4-(Arylhydrazino)coumarins reacted with 4-chlorobenzaldehyde in the presence of piperidine acetate to give the corresponding 2-aryl-3-(4-chlorophenyl)[1]benzopyrano[4,3-b]pyrazol-4-ones. The reaction of 4-(4-bromophenylhydrazino)-coumarin with 4-chlorobenzaldehyde in the presence of piperidine acetate and an excess of piperidine gave 2-(4-bromophenyl)-5-(4-chlorophenyl)-3-(2-hydroxyphenyl)-4-(piperidinocarbonyl)pyrazole, but the reaction of phenyl- and 4-(2-chlorophenylhydrazino)coumarins with 4-chlorobenzaldehyde gave 1-aryl-5-(4-chlorophenyl)-3-(2-hydroxyphenyl)-4-(1-piperidino)carbonyl-4,5-dihydropyrazoles.
Similar content being viewed by others
References
I. Strakova, M. Petrova, S. Belyakov, and A. Strakovs, Khim. Geterotsikl. Soed., 1827 (2003). [Chem. Heterocycl. Comp., 39, 1608 (2003)].
I. Strakova. M. Petrova, S. Belyakov, and A. Strakovs, Khim. Geterotsikl. Soed., 660 (2006). [Chem. Heterocycl. Comp., 42, 574 (2006)].
I. Strakova. M. Petrova, S. Belyakov, and A. Strakovs, Khim. Geterotsikl. Soed., 935 (2007). [Chem. Heterocycl. Comp., 43, 793 (2007)].
M. Čačič, M. Trkovnik, and E. Has-Schön, J. Heterocycl. Chem., 40, 833 (2002).
V. V. Mulwad and J. M. Shirodkar, Indian. J. Chem., 41B, 1263 (2002).
V. Colotta, L. Cecchi, F. Melani, G. Filaccioni, C. Martini, S. Gelli, and A. Lucacchini, J. Pharm. Sci., 80, 276 (1991).
A. A. Shestopalov and V. P. Litvinov, Izv. AN, Ser. Khim., 968 (2005).
I. A. Khan, M. V. Kukarni, M. Gopal, M. S. Shahabuddin, and C.-M. Sun, Bioorg. Med. Chem. Lett., 15, 3584 (2005).
E. M. Becalli, A. Contini, and P. Trimerco, Tetrahedron. Lett., 45, 3447 (2004).
F. H. Havaldar and S. S. Bhise, Indian J. Heterocycl. Chem., 13, 15 (2003).
V. L. Savel’ev, O. L. Samsonov, V. P. Lezina, V. S. Troitskaya, I. I. Kozlovskii, A. Beshimov, and M. M. Kozlovskaya. Khim.-farm. Zh., 37, No. 9, 25 (2003).
F. Al-Omran, A.-Z. A. Elassar, and A. A. El-Khair, J. Heterocycl. Chem., 40, 249 (2003).
A. Alberol, L. Calvo, A. Gonzlez-Ortega, E. F. Encabo, and M. C. Sanudo, Synthesis, 194 (2001).
J. A. Frogget, M. H. Hockley, and R. B. Titman, J. Chem. Res. (S), 30 (1997).
A. Altomare, M. Burla, M. Camalii, G. Cascarano, C. Giacovazzo, A. Guagliardi, A. Moliterni, and R. Spagna, J. Appl. Crystallogr., 32, 115 (1999).
S. Mackay, C. Edwards, A. Henderson, C. J. Gilmore, N. Stewart, K. Shankland, and A Donald, maXus Computer Program for the Solution and Refinement of Crystal Structures, Bruker Nonius, The Netherlands; MacSci., Japan, 1999.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 11, 1643–1650, November, 2009.
Rights and permissions
About this article
Cite this article
Strakova, I., Strakovs, A., Petrova, M. et al. Synthesis and reactions of 4-(arylhydrazino)coumarins. Chem Heterocycl Comp 45, 1319–1324 (2009). https://doi.org/10.1007/s10593-010-0428-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-010-0428-y