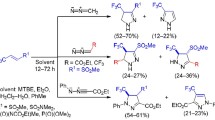
Esters of 2-arylcyclopropanecarboxylic acids react with nitrous acid generated in situ with regioselective insertion of the nitrosyl cation into the cyclopropane ring. Depending on the substrate/nitrosylating agent ratio, the reaction proceeds with the formation of either aryl-substituted 3-ethoxycarbonyl-4,5-dihydroisoxazoles or the corresponding isoxazoles. The nature and position of the substituents in the aromatic ring of the starting 2-arylcyclopropanecarboxylic acid esters affect the reaction rate but have no effect on the regioselectivity of the attack by the nitrosyl cation on the three-membered ring. A dependence of the reactivity of isomeric substrates on their stereochemistry and position of the nitro group in the aromatic ring is noted for 2- and 4-nitrophenyl derivatives of esters of cis- and trans-2-arylcyclopropanecarboxylic acids.
Similar content being viewed by others
References
T. Tatee, S. Kurashige, A. Shiozawa, K. Narita, M. Takei, S. Ito, H. Migazaki, H. Yamanaka, M. Mirugaki, T. Sakamado, and H. Fukada, Chem. Pharm. Bull., 34, 1634 (1986).
V. Venkateshwarlu, A. Krishnamurthy, and C. J. Rao, Indian J. Chem., 27B, 565 (1989).
B. H. Lipshutz, Chem. Rev., 86, 795 (1986).
P. G. Baraldi, A. Barco, S. Benetti, G. P. Pollini, and D. Simoni, Synthesis, 857 (1987).
P. A. Wade and J. F. Beremak, J. Org. Chem., 52, 2973 (1987).
D. P. Curran, S. A. Scanga, and C. J. Fenk, J. Org. Chem., 49, 3474 (1984).
Yu. S. Shabarov, L. G. Saginova, and R. A. Gazzaeva, Khim. Geterotsikl. Soedin., 738 (1983). [Chem. Heterocycl. Comp., 19, 589 (1983)].
S. S. Mochalov, Ya. I. Kuz’min, A. N. Fedotov, E. V. Trofimova, R. A. Gazzaeva, Yu. S. Shabarov, and N. S. Zefirov, Zh. Org. Khim., 34, 1379 (1998).
O. B. Bondarenko, A. Yu. Gavrilova, L. G. Saginova, N. V. Zyk, and N. S. Zefirov, Izv. Akad. Nauk, Ser. Khim., 741 (2003).
R. A. Gazzaeva, S. S. Mochalov, B. P. Archegov, and N. S. Zefirov, Khim. Geterotsikl. Soedin., 302 (2005). [Chem. Heterocycl. Comp., 41, 272 (2005)].
N. Ichinose, K. Mizuno, T. Tamai, and Y. Otsuji, Chem. Lett., 233 (1988).
K. Mizuno, N. Ichinose, T. Tamai, and Y. Otsuji, J. Org. Chem., 57, 4669 (1992).
S.-T. Lin, S.-H. Kuo, and F.-M. Yang, J. Org. Chem., 62, 5229 (1997).
A. Z. Kadzhaeva, E. V. Trofimova, R. A. Gazzaeva, A. N. Fedotov, and S. S. Mochalov, Vestn. Moskovsk. Gos. Univ., Ser. 2, Khimiya, 50, 35 (2009).
H.-U. Reissig and R. Zimmer, Chem. Rev., 103, 1151 (2003).
M. Yu and B. L. Pagenkopf, Tetrahedron, 61, 321 (2005).
A. M. Bernard, A. Frongia, P. P. Piras, F. Secci, and M. Spiga, Org. Lett., 7, 4565 (2005).
A. M. Bernard, A. Frongia, F. Secci, G. Delogu, J. Ollivier, P. P. Piras, and J. Salaün, Tetrahedron, 59, 9433 (2003).
M. Yu and B. L. Pagenkopf, Org. Lett., 5, 5099 (2003).
J. V. Girvello, J. Org. Chem., 46, 3056 (1981).
M. A. Weidner-Wells, S. A. Fraga-Spano, and I. J. Turchi, J. Org. Chem., 63, 6319 (1998).
S. S. Mochalov, R. A. Gazzaeva, A. N. Fedotov, E. V. Trofimova, I. V. Trushkov, and N. S. Zefirov, Zh. Org. Khim., 40, 1146 (2004).
A. Burger and W. L. Yost, J. Am. Chem. Soc., 70, 2198 (1948).
V. Grinshtein and M. Andersone, Izv. Akad. Nauk LatvSSR, Ser. Khim., 106 (1963).
A. Nakamura, A. Konishi, R. Tsujitani, M. Kudo, and S. Otsuka, J. Am. Chem. Soc., 100, 3449 (1978).
A. Dupin and R. Fraisse-Jullien, Bull. Soc. Chim. Fr., 1993 (1964).
A. N. Fedotov, E. V. Trofimova, S. S. Mochalov, and Yu. S. Shabarov, Zh. Org. Khim., 24, 1413 (1988).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 5, pp. 753–765, May, 2009.
Rights and permissions
About this article
Cite this article
Kadzhaeva, A.Z., Trofimova, E.V., Fedotov, A.N. et al. Reaction of esters of 2-arylcyclo-propanecarboxylic acids with nitrous acid. Synthesis of aryl-substituted 3-ethoxycarbonyl-4,5-dihydroisoxazoles and 3-ethoxycarbonylisoxazoles. Chem Heterocycl Comp 45, 595–605 (2009). https://doi.org/10.1007/s10593-009-0315-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-009-0315-6