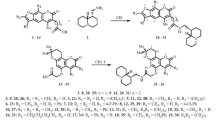
New 4-chloromethyl-6-hydroxycoumarins have been obtained by the condensation of hydroquinone derivatives with 4-chloroacetoacetic ester, and have been used for the synthesis of 4-(2-benzofuryl)-6-hydroxycoumarins. Alkylation and acylation of the phenolic hydroxyl of the synthesized 4-(2-benzo-furyl)coumarins have been investigated.
Similar content being viewed by others
References
G. V. M. Sharma, J. J. Reddy, P. S. Lakshmi, and P. R. Krishna, Tetrahedron Lett., 46, 6119 (2005).
S. D. Joshi and R. N. Usgaonkar, Indian J. Chem. 21B, 399 (1982).
F. Leonetti, A. Favia, A. Rao, R. Aliano, A. Paluszcak, R. W. Hartmann, and A. Carotti, J. Med. Chem., 47, 6792 (2004).
R. Dey, J. Indian Chem. Soc., 11, 635 (1934).
P. Waykole and R. N. Usgaonkar, Indian J. Chem. 21B, 707 (1982).
Y. Fall, L. Santana, M. Teijeira, and E. Uriarte, Heterocycles, 41, 647 (1995).
A. P. Silva, H. Q. N. Gunaratne, P. L. M. Lynch, A. J. Patty, and G. L. Spence, J. Chem. Soc., Perkin Trans. 2, 1611 (1993).
G. Renzi, A. Scozzafava, and C. T. Supuran, Bioorg. Med. Chem. Lett., 10, 673 (2000).
A. Long, J. Parrick, and R. J. Hodgkiss, Synthesis, 709 (1991).
S. P. Bondarenko, M. S. Frasinyuk, and V. P. Khilya, Khim. Prir. Soedin., 206 (2003).
M. S. Frasinyuk, S. P. Bondarenko, and V. P. Khilya, Khim. Prir. Soedin., 117 (2006).
S. Hanmantgad, M. Kulkarnti, V. Patil, P. Diwan, and D. Kulkarnti, Indian J. Chem., 25B, 779 (1986).
A. R. Deshpande, U. K. Joshi, and M. V. Paradkar, Indian J. Chem., 27B, 524 (1988).
K. Y. Anklekar, C. D. Lakkannavar, G. M. Kulkarni, and M. V. Kulkarni, Indian J. Chem., 42B, 1548 (2003).
M. Ghate, D. Manohar, V. Kulkarni, R. Shobha, and S. Y. Kattimani, Eur. J. Med. Chem., Chim. Ther., 38, 297 (2003).
I. A. Khan and M. V. Kulkarni, Indian J. Chem., 38B, 491 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 3, pp. 361–369, March, 2009.
Rights and permissions
About this article
Cite this article
Frasinyuk, M.S., Bondarenko, S.P. & Khilya, V.P. Synthesis and properties of 4-chloromethyl-6-hydroxy-coumarins and 4-(2-benzofuryl)-6-hydroxycoumarins. Chem Heterocycl Comp 45, 290–296 (2009). https://doi.org/10.1007/s10593-009-0275-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-009-0275-x